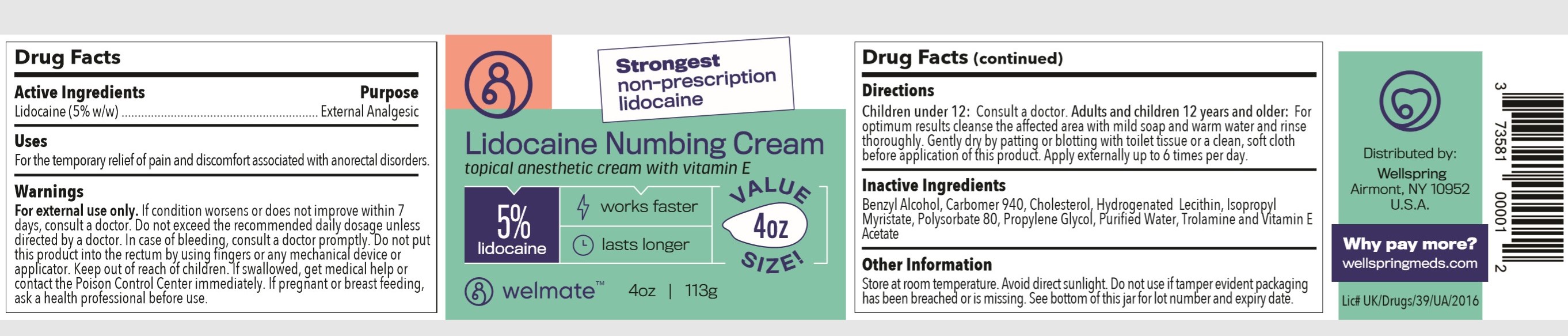
Warnings:
- For external use only. If condition worsens or does not improve within 7 days, consult a doctor.
When using this product,
- Do not exceed the recommended daily dosage unless directed by a doctor.
- In case of bleeding, consult a doctor promptly.
- Do not put this product into the rectum by using fingers or any mechanical device or applicator.
- Keep out of reach of children.
- If swallowed, get medical help or contact the Poison Control Center immediately.
- If pregnant or breast feeding, ask a health professional before use.
Directions:
Children under 12: Consult a doctor.
Adults and children 12 years and older: For optimum results cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a clean, soft cloth before application of this product. Apply externally up to 6 times per day.
Inactive Ingredients
Benzyl Alcohol, Carbomer 940, Cholesterol, Hydrogenated Lecithin, Isopropyl Myristate, Polysorbate 80, Propylene Glycol, Purified Water, Trolamine and Vitamin E Acetate