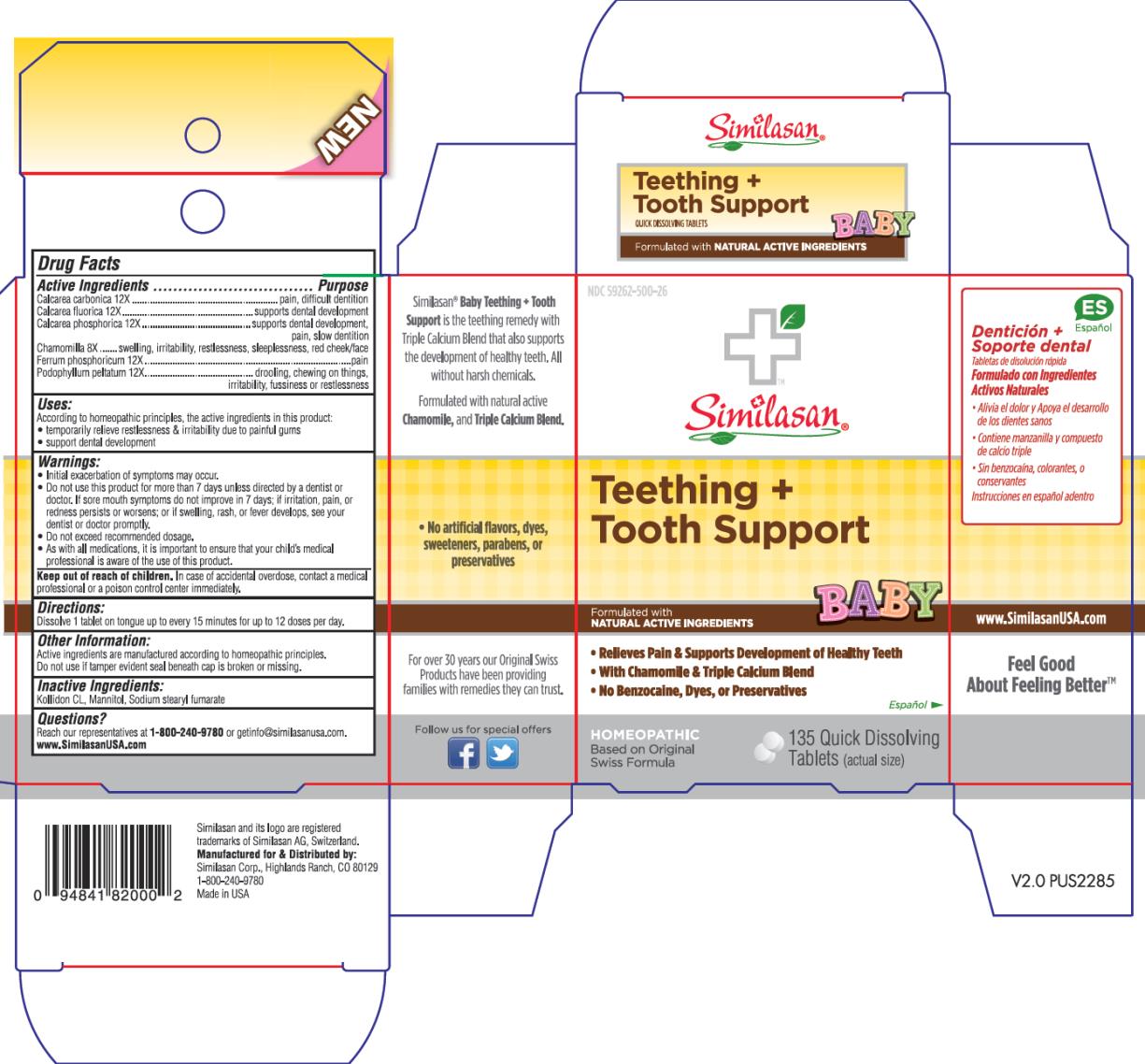
BABY TEETHING PLUS TOOTH SUPPORT- oyster shell calcium carbonate, crude, calcium fluoride, tribasic calcium phosphate, matricaria recutita, ferrosoferric phosphate, and podophyllum tablet, orally disintegrating
Similasan Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
Calcarea carbonica 12X
Purpose
pain, difficult dentition
Active Ingredient
Calcarea fluorica 12X
Purpose
supports dental development
Active Ingredient
Calcarea phosphorica 12X
Purpose
supports dental development, pain, slow dentition
Active Ingredient
Chamomilla 8X
Purpose
swelling, irritability, restlessness, sleeplessness, red cheek/face
Active Ingredient
Ferrum phosphoricum 12X
Purpose
fussiness or restlessness
Active Ingredient
Podophyllum peltatum 12X
Purpose
drooling, chewing on things, irritability
Uses:
According to the homeopathic principles, the active ingredients in this product:
- temporarily relieve restlessness & irritability due to painful gums
- support dental development
Warnings:
- Initial exacerbation of symptoms may occur.
- Do not use this product for more than 7 days unless directed by a dentist or doctor. If sore mouth symptoms do not improve in 7 days; if irritation, pain, or redness persists or worsens; or if swelling, rash, or fever develops, see your dentist or doctor promptly.
- Do not exceed recommended dosage.
- As with all medications, it is important to ensure that your child’s medical professional is aware of the use of this product.
Keep out of reach of children.
In case of accidental overdose, contact a medical professional or a poison control center immediately.
Directions:
Dissolve 1 tablet on tongue up to every 15 minutes for up to 12 doses per day.
Other Information:
Active ingredients are manufactured according to homeopathic principles.
Do not use if tamper evident seal beneath cap is broken or missing.
Inactive Ingredients:
Kollidon CL, Mannitol, Sodium stearyl fumarate
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com.
www.SimilasanUSA.com
PRINCIPAL DISPLAY PANEL
NDC 59262-500-26
Similasan®
Teething +
Tooth Support
BABY
135 Quick Dissolving
Tablets