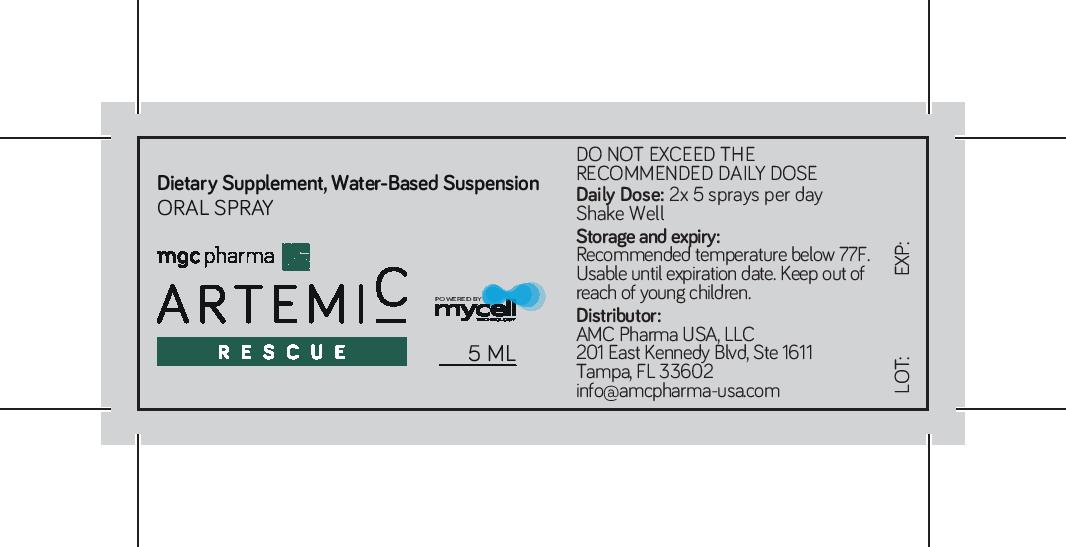
Water-based suspension, dietary supplement Oral Spray
Curcuma Longa Extract, Boswellia SerrataExtract, Antioxidant Artemisinin,Vitamin C (L-Ascorbic Acid)
Water, Vegetable Castor Oil, Natural Propanediol, DL-Alpha-Tocopherol
5 sprays provides 1 ml of ArtemiC. Servings per container: 4
Shake well before using. Adults apply 5 sprays, twice daily in the mouth, morning and evening for 2 consecutive days.preferably at least half hour before eating.
Keep out of reach of children. Not intended for pregnant or nursing woman. Discontinue use or consult your doctor if any adverse reactions occur. Do not use if inner bottle or spray cap is broken, or shows sign of tampering.
Keep Out of Reach of Children.
Store in a dry, cool place and avoid heat and direct sunlight. Do not exceed temperatures above 77 deg F. Usable until expiration date. Once opened, use witjin 1 month. Do not freeze.
Adults apply 5 sprays, twice daily, into mouth.
Dietary supplement to support the immune system.
NDC 83278-001-05

Bottle label NDC 83278-001-05