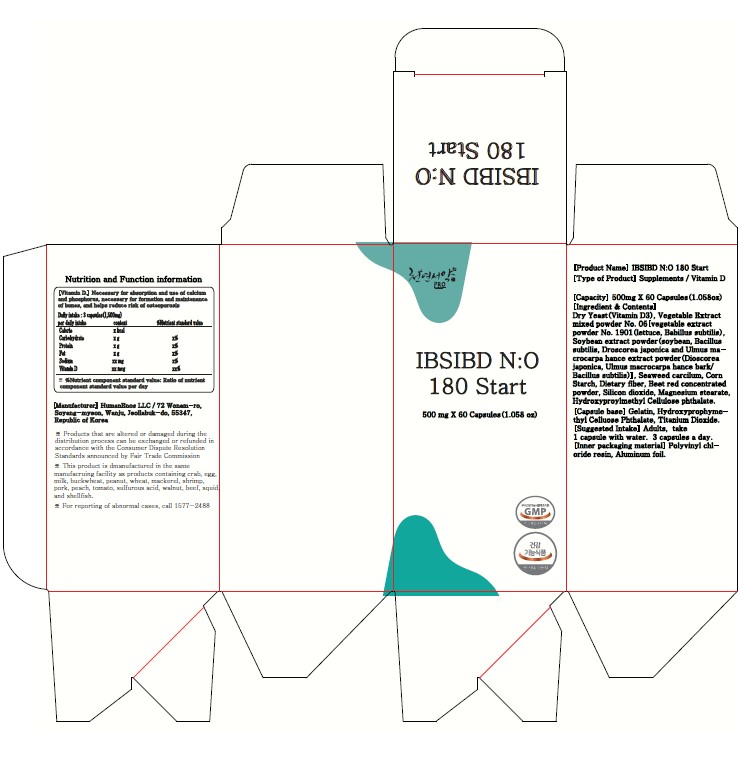
IBSIBD NO 180 START (HELPS IMPROVE INTESTINAL HEALTHY AND IMMUNITY, LETTUCE AND SOYBEAN FERMENTED POWDER)- nitric oxide, vitamin d3, glutathione capsule
HumanEnos LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
These highlights do not include all the information needed to use. See full prescribing information.
500mg x 60 / 1.058oz
KEEP OUT OF THE REACH OF CHILDREN.
Inactive ingredients:
Water
Active Ingredients
Nitric Oxide
IBSIBD N:O 180 Start (Helps improve intestinal healthy and immunity/ Lettuce and Soybean fermented powder)