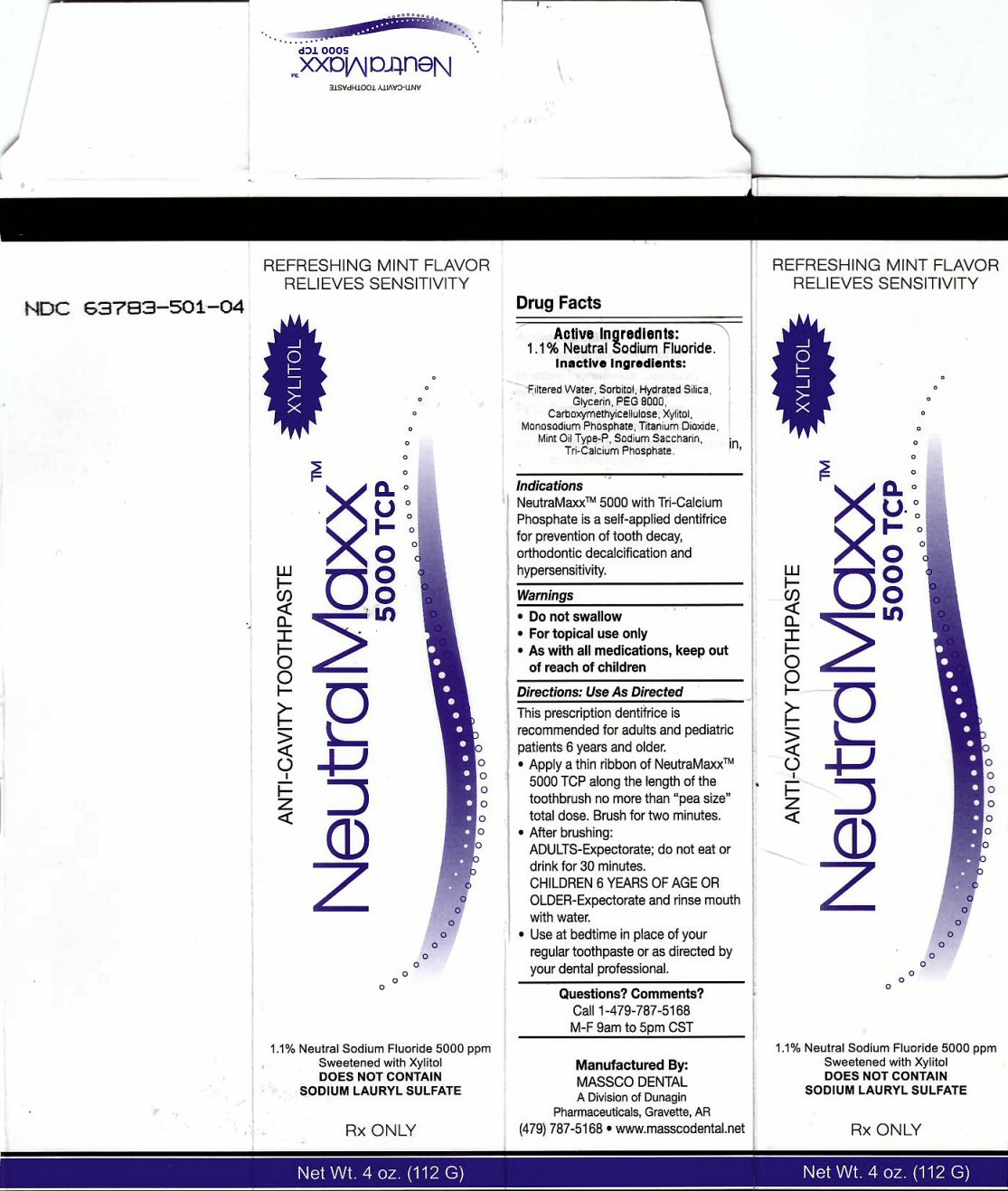
Indications and Usage Section
NeutraMaxxtm 5000 with Tri-Calcium Phosphate is a self applied dentifrice for prevention of tooth decay, orthodontic decalcification and hypersensitivity
Directions: Use As Directed Section
This prescription dentifrice is recommended for adults and pediatric patients 6 years and older
Apply a thin ribbon of NeutraMaxxtm 5000 TCP along the length of the toothbrush no more than "pea size" total dose. Brush for two minutes.
After brushing ADULTS - Expectorate, do not eat for 30 minutes. CHILDREN 6 YEARS OF AGE OR OLDER - Expectorate and rinse mouth with water. Use at bedtime in place of your regular toothpaste or as directed by your dental professional.
Inactive Ingredient Section
Inactive Ingredients: Deionized Water, Sorbitol, Hydrated Silica, PEG, Carboxymethylcellulose, Xylitol, MonoSodium Phosphate, Titanium Dioxide, Mint Flavor, Sodium Saccharin, Tri-Calcium Phosphate
Package Label
REFRESHING MINT FLAVOR RELIEVES SENSITIVITY
ANTI-CAVITY TOOTHPASTE NeutraMaxx tm 5000 TCP XYLITO1.1% Neutral Sodium Fluoride 5000 ppm Sweetened with Xylitol
DOES NOT CONTAIN SODIUM LAURYL SULFATE RX ONLY Net wt. 4 oz (112 G)
Manufactured By: MASSCO DENTAL A Division of Dunagin Pharmaceuticals, Gravette, AR (479) 787-5168 www.masscodental.net
res