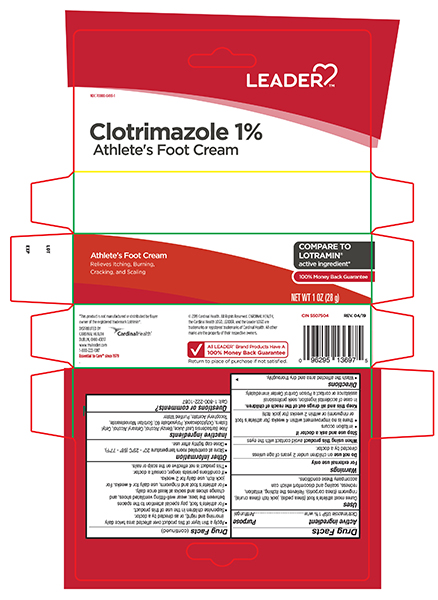
Uses
Cures athlete's foot (tinea pedis), jock itch (tinea cruris), ringworm (tinea corporis). Relieves the itching, irritation, redness, scaling and chafing associated with jock itch.
Warnings
Directions
<This product is not effective on the scalp or nails.
- Clean the affected area and dry thoroughly.
- Apply a thin layer of this product over affected area twice daily (morning and night), or as directed by a doctor.
- Supervise children in the use of this product.
- use daily for 2 weeks.
- If conditions persists longer, consult a doctor.>
Other information
- Store at controlled room temperature 20°-25°C (68°-77°F).
- Close cap tightly after use.
Inactive ingredients
aloe vera, benzyl alcohol, cetearyl alcohol, 2-octyldodecanol, polysorbate 60, sorbitan monostearate, spermwax, vitamin E, water
Questions?
For Questions or comments or to report an undesired reaction or side effect, please call 1-800-222-1222