FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Olopatadine hydrochloride nasal spray is indicated for the relief of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 6 years of age and older.
2 DOSAGE AND ADMINISTRATION
2.1 Adults and Adolescents Twelve Years of Age and Older: The recommended dosage is two sprays per nostril twice daily.
2.2 Pediatric Patients Six to Eleven Years Of Age: The recommended dosage is one spray per nostril twice daily.
2.3 Administration Information
Administer olopatadine hydrochloride nasal spray by the nasal route only.
Priming: Before initial use, prime olopatadine hydrochloride nasal spray by releasing 5 sprays or until a fine mist appears. The correct amount of medication cannot be assured before the initial priming.
Re-priming (as needed): When olopatadine hydrochloride nasal spray has not been used for more than 7 days, re-prime by releasing 2 sprays. Avoid spraying olopatadine hydrochloride nasal spray into the eyes.
Discard Instructions: Discard nasal device after 240 sprays (enough for 30 days of dosing) have been used even though the bottle is not completely empty. The correct amount of medication cannot be assured after 240 sprays have been used.
3 DOSAGE FORMS AND STRENGTHS
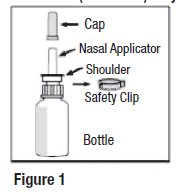
Nasal spray: 665 mcg olopatadine hydrochloride per spray supplied in a white plastic bottle with a metered-dose manual spray pump, a white nasal applicator, a white overcap, and a safety clip.
5 WARNINGS AND PRECAUTIONS
5.1 Local Nasal Effects
Epistaxis and Nasal Ulceration: In placebo-controlled clinical trials (vehicle nasal spray) of 2 weeks to 12 months duration, epistaxis and nasal ulcerations were reported [see Adverse Reactions (6)].
Nasal Septal Perforation:
Three placebo-controlled long term (12 months) safety trials (vehicle nasal spray) were conducted. In the first safety trial, patients were treated with an investigational formulation of olopatadine hydrochloride nasal spray containing povidone (not the commercially marketed formulation) or a vehicle nasal spray containing povidone. Nasal septal perforations were reported in one patient treated with the investigational formulation of olopatadine hydrochloride nasal spray and 2 patients treated with the vehicle nasal spray. In the second safety trial with olopatadine hydrochloride nasal spray, which does not contain povidone, there were no reports of nasal septal perforation. In the third safety trial, one patient exposed to the 3.7 pH vehicle nasal spray (containing no povidone) reported a nasal septal perforation [see Adverse Reactions (6)].
Before starting olopatadine hydrochloride nasal spray, conduct a nasal examination to ensure that patients are free of nasal disease other than allergic rhinitis. Perform nasal examinations periodically for signs of adverse effects on the nasal mucosa and consider stopping olopatadine hydrochloride nasal spray if patients develop nasal ulcerations.
5.2 Somnolence and Impaired Mental Alertness
In clinical trials, the occurrence of somnolence has been reported in some patients taking olopatadine hydrochloride nasal spray [see Adverse Reactions (6)]. Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness and motor coordination such as driving or operating machinery after administration of olopatadine hydrochloride nasal spray. Concurrent use of olopatadine hydrochloride nasal spray with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
6 ADVERSE REACTIONS
The most clinically significant adverse reactions described in other sections of labeling include;
- •
- Epistaxis, Nasal Ulceration, and Nasal Septal Perforation [see Warnings and Precautions (5.1)]
- •
- Somnolence and Impaired Mental Alertness [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
In a 12-month, placebo-controlled, safety trial (vehicle nasal spray), 890 patients 12 years of age and older with perennial allergic rhinitis were randomized to treatment with olopatadine hydrochloride nasal spray two sprays per nostril twice daily (445 patients) or vehicle nasal spray (445 patients). In the olopatadine hydrochloride nasal spray and vehicle nasal spray groups, 72% and 74% of patients, respectively, completed the trial. Overall, 7% and 5%, respectively, discontinued study participation due to an adverse reaction. The most frequently reported adverse reaction was epistaxis, which occurred in 25% of patients treated with olopatadine hydrochloride nasal spray and 28% in patients treated with vehicle nasal spray. Epistaxis resulted in discontinuation of 0.9% of patients treated with olopatadine hydrochloride nasal spray and 0.2% of patients treated with vehicle nasal spray. Nasal ulcerations occurred in 10% of patients treated with olopatadine hydrochloride nasal spray and 9% of patients treated with vehicle nasal spray. Nasal ulcerations resulted in discontinuation of 0.4% of patients treated with olopatadine hydrochloride nasal spray and 0.2% patients treated with vehicle nasal spray. There were no patients with nasal septal perforation in either treatment group. Somnolence was reported in 1 patient treated with olopatadine hydrochloride nasal spray and 1 patient treated with vehicle nasal spray. Weight increase was reported in 6 patients treated with olopatadine hydrochloride nasal spray and 1 patient treated with vehicle nasal spray. Depression or worsening of depression occurred in 9 patients treated with olopatadine hydrochloride nasal spray and in 5 patients treated with vehicle nasal spray. Three patients, 2 of whom had preexisting histories of depression, who received olopatadine hydrochloride nasal spray were hospitalized for depression compared to none who received vehicle nasal spray.
In a second 12-month, placebo-controlled, safety trial (vehicle nasal spray), 459 patients 12 years of age and older with perennial allergic rhinitis were treated with two sprays per nostril of an investigational formulation of olopatadine hydrochloride nasal spray containing povidone (not the commercially marketed formulation) and 465 patients were treated with 2 sprays of a vehicle nasal spray containing povidone. Nasal septal perforations were reported in one patient treated with the investigational formulation of olopatadine hydrochloride nasal spray and 2 patients treated with the vehicle nasal spray. Epistaxis was reported in 19% of patients treated with the investigational formulation of olopatadine hydrochloride nasal spray and 12% of patients treated with vehicle nasal spray. Somnolence was reported in 3 patients treated with the investigational formulation of olopatadine hydrochloride nasal spray compared to 1 patient treated with vehicle nasal spray. Fatigue was reported in 5 patients treated with the investigational formulation of olopatadine hydrochloride nasal spray compared to 1 patient treated with vehicle nasal spray.
In a third 3-arm, 12-month placebo-controlled, safety trial (vehicle nasal spray), conducted post approval, 1,026 patients 12 years of age and older with perennial allergic rhinitis were randomized to treatment with olopatadine hydrochloride nasal spray (343 patients), a 3.7 pH vehicle nasal spray (341 patients), or a 7.0 pH vehicle nasal spray (342 patients). All treatments were administered as two sprays per nostril, twice daily. Overall, 5% of olopatadine hydrochloride nasal spray patients, 2% of 3.7 pH vehicle patients and 3% of 7.0 pH vehicle patients discontinued due to adverse reactions. The most frequently reported adverse reaction was epistaxis, which occurred in 24% of patients treated with olopatadine hydrochloride nasal spray, 20% of patients treated with 3.7 pH vehicle nasal spray, and 23% of patients treated with 7.0 pH vehicle nasal spray. Epistaxis resulted in the discontinuation of 2 patients treated with olopatadine hydrochloride nasal spray and 1 patient treated with 7.0 pH vehicle nasal spray. Nasal septal perforation was reported for one patient treated with the 3.7 pH vehicle nasal spray. Nasal ulcerations occurred in 9% of patients treated with olopatadine hydrochloride nasal spray, 8% of patients treated with 3.7 pH vehicle nasal spray, and 9% of patients treated with 7.0 pH vehicle nasal spray. Nasal ulceration resulted in the discontinuation of 1 patient treated with olopatadine hydrochloride nasal spray. Hyposmia and anosmia were each reported by one patient treated with olopatadine hydrochloride nasal spray. Neither somnolence nor weight loss was reported. Depression occurred in 3 patients treated with olopatadine hydrochloride nasal spray, 2 patients treated with 3.7 pH vehicle nasal spray, and 3 patients treated with 7.0 pH vehicle nasal spray.
There were no long-term clinical trials in children below 12 years of age.
6.2 Post-Marketing Experience
During the post approval use of olopatadine hydrochloride nasal spray, the following adverse reactions have been identified. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The most common adverse reactions reported include dizziness, dysgeusia, epistaxis, headache, nasal discomfort, oropharyngeal pain, and somnolence. Additionally, hyposmia and anosmia have been reported with the use of olopatadine hydrochloride nasal spray.
7 DRUG INTERACTIONS
Formal drug-drug interaction studies were not conducted for olopatadine hydrochloride nasal spray. Drug interactions with inhibitors of liver enzymes are not anticipated because olopatadine is eliminated predominantly by renal excretion. Drug interactions involving P450 inhibition and plasma protein binding are also not expected. [See Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from postmarketing experience with antihistamines, with similar mechanism of action to olopatadine hydrochloride nasal spray, have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. However, there are no published human data specific to olopatadine hydrochloride nasal spray. In animal reproductive studies, oral administration of olopatadine hydrochloride to pregnant rats and rabbits caused a decrease in the number of live fetuses at maternal doses approximately 110 and 1,460 times the maximum recommended human daily intranasal dose (MRHDID) on a mg/m2 basis, respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4%, and 15% to 20%, respectively.
Data
Animal Data
In an oral embryo-fetal development study, pregnant rabbits were dosed throughout the period of organogenesis at doses up to 400 mg/kg/day. A decrease in the number of live fetuses was observed at 400 mg/kg/day (1,460 times the MRHDID, on a mg/m2 basis).
In an oral embryo-fetal development study, pregnant rats were dosed throughout the period of organogenesis at doses up to 600 mg/kg/day. Maternal toxicity, producing death and reduced maternal body weight gain was observed at 600 mg/kg/day (approximately 1,100 times the MRHDID on a mg/m2 basis). Olopatadine produced cleft palate at 60 mg/kg/day (approximately 110 times the MRHDID on a mg/m2 basis) and decreased embryo-fetal viability and reduced fetal weight in rats at 600 mg/kg/day (approximately 1,100 times the MRHDID on a mg/m2 basis).
In peri-/postnatal toxicity studies, pregnant rats received oral doses of olopatadine up to 600 mg/kg/day during late gestation and throughout the lactation period. Olopatadine produced decreased neonatal survival at 60 mg/kg/day (approximately 110 times the MRHDID on a mg/m2 basis) and reduced body weight gain in pups at 4 mg/kg/day (approximately 7 times the MRHDID on a mg/m2 basis). These effects appeared attributable to exposure of pups via the milk as demonstrated in a cross-fostered study in which pups of untreated dams cross-fostered to dams treated with 60 mg/kg/day olopatadine orally during the lactation period exhibited decreased body weight gain.
8.3 Lactation
Risk Summary
There are no data on the presence of olopatadine in human milk, the effects on the breastfed infant, or the effects on milk production. Although orally administered olopatadine is present in rat milk, there is no information about nasally administered olopatadine.
It is not known whether topical nasal administration could result in sufficient systemic absorption to produce detectable quantities in human breast milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for olopatadine hydrochloride nasal spray and any potential adverse effects on the breast fed infant from olopatadine hydrochloride nasal spray or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of olopatadine hydrochloride nasal spray for the relief of symptoms of seasonal allergic rhinitis have been established in pediatric patients aged 6 years and older.
The safety and effectiveness of olopatadine hydrochloride nasal spray in pediatric patients 6 to 11 years of age are supported by 3 vehicle-controlled 2-week studies in 870 patients [see Adverse Reactions (6.1)]. Doses studied included one and two sprays per nostril twice daily. One of these studies evaluated the safety of olopatadine hydrochloride nasal spray at doses of one and two sprays per nostril twice daily in 1,188 patients, of which 298 patients were exposed to olopatadine hydrochloride nasal spray 1 spray, and 297 patients were exposed to vehicle 1 spray. In this study, the incidence of epistaxis with olopatadine hydrochloride nasal spray use was 5.7% [see Adverse Reactions (6.1), Clinical Studies (14)].
The safety and effectiveness of olopatadine hydrochloride nasal spray in pediatric patients aged 12 years and older are supported by 3 randomized, double blind, parallel group, multicenter, placebo-controlled clinical trials of 2 weeks duration in adult and adolescent patients [see Clinical Studies (14)]. In these studies, the incidence of epistaxis with olopatadine hydrochloride nasal spray use in 587 patients was 3.2% [see Adverse Reactions (6.1)].
The safety and effectiveness of olopatadine hydrochloride nasal spray have not been established in pediatric patients under 6 years of age.
The safety of olopatadine hydrochloride nasal spray at a dose of one spray per nostril twice daily was evaluated in one 2-week vehicle-controlled study in 132 pediatric patients 2 to 5 years of age with allergic rhinitis. In this trial, 66 patients were exposed to olopatadine hydrochloride nasal spray. The most common (greater than 1.0%) adverse reactions reported were diarrhea (9.1%), epistaxis (6.1%), rhinorrhea (4.5%), bitter taste (3.0%), and wheezing (3.0%). Diarrhea was reported more frequently (9.1%) in patients 2 to 5 years of age than 6 to 11 year old age group (< 1%).
8.5 Geriatric Use
Clinical studies of olopatadine hydrochloride nasal spray did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
10 OVERDOSAGE
Symptoms of antihistamine overdose may include drowsiness in both adults and children. Agitation and restlessness followed by drowsiness may also occur in children. There is no known specific antidote to olopatadine hydrochloride nasal spray. Should overdose occur, symptomatic or supportive treatment is recommended, taking into account any concomitantly ingested medications.
For additional information about overdose treatment, call a poison control center (1-800-222-1222).
11 DESCRIPTION
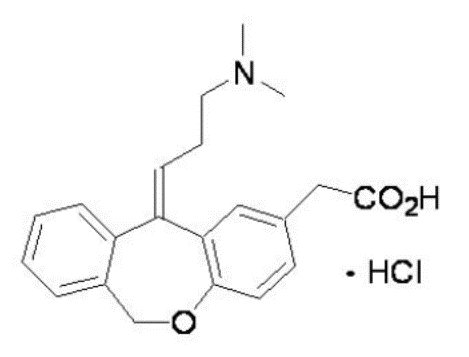
Olopatadine Hydrochloride Nasal Spray, 665 micrograms (mcg) is a metered-spray solution for intranasal administration. Olopatadine hydrochloride, the active component of Olopatadine Hydrochloride Nasal Spray, is a white, water-soluble crystalline powder. The chemical name for olopatadine hydrochloride is (Z)-11-[3-(dimethylamino)propylidene]-6,11-dihydrodibenz [b,e]oxepin-2-acetic acid hydrochloride. It has a molecular weight of 373.88, and its molecular formula is C21H23NO3 • HCl with the following chemical structure:
Olopatadine Hydrochloride Nasal Spray contains 0.6% w/v olopatadine (base) in a nonsterile aqueous solution with pH of approximately 3.7. After initial priming (5 sprays), each metered spray from the nasal applicator delivers 100 microliters of the aqueous solution containing 665 mcg of olopatadine hydrochloride, which is equivalent to 600 mcg of olopatadine (base) [see Dosage Forms and Strengths (3)]. Olopatadine Hydrochloride Nasal Spray also contains benzalkonium chloride (0.01%), dibasic sodium phosphate, edetate disodium, sodium chloride, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Olopatadine is a histamine H1 -receptor antagonist. The antihistaminic activity of olopatadine has been documented in isolated tissues, animal models, and humans.
12.2 Pharmacodynamics
Cardiac Electrophysiology: In a placebo-controlled cardiovascular safety study, 32 healthy volunteers received 20 mg oral solution of olopatadine twice daily for 14 days (8-fold greater daily dose than the recommended daily nasal dose). The mean QTcF (QT corrected by Fridericia’s correction method for heart rate) change from baseline was -2.7 msec and -3.8 msec for olopatadine, and placebo, respectively. In this study, 8 subjects treated with olopatadine had a QTcF change from baseline of 30 to 60 msec, 1 subject had a QTcF change from baseline greater than 60 msec, and no subjects had QTcF values greater than 500 msec. Eight subjects treated with placebo had a QTcF change from baseline of 30 to 60 msec, no subjects had a QTcF change from baseline greater than 60 msec, and no subjects had QTcF values greater than 500 msec. In a 12-month study in 429 perennial allergic rhinitis patients treated with olopatadine hydrochloride nasal spray 2 sprays per nostril twice daily, no evidence of any effect of olopatadine hydrochloride on QT prolongation was observed.
12.3 Pharmacokinetics
The pharmacokinetic properties of olopatadine were studied after administration by the nasal, oral, intravenous, and topical ocular routes. Olopatadine exhibited linear pharmacokinetics across the routes studied over a large dose range.
Absorption:
Healthy Subjects: Olopatadine was absorbed with individual peak plasma concentrations observed between 30 minutes and 1 hour after twice daily intranasal administration of olopatadine hydrochloride nasal spray. The mean (± SD) steady-state peak plasma concentration (Cmax) of olopatadine was 16.0 ± 8.99 ng/mL. Systemic exposure as indexed by area under the curve (AUC0-12) averaged 66.0 ± 26.8 ng·h/mL. The average absolute bioavailability of intranasal olopatadine is 57%. The mean accumulation ratio following multiple intranasal administration of olopatadine hydrochloride nasal spray was about 1.3.
Seasonal Allergic Rhinitis (SAR) Patients: Systemic exposure of olopatadine in SAR patients after twice daily intranasal administration of olopatadine hydrochloride nasal spray was comparable to that observed in healthy subjects. Olopatadine was absorbed with peak plasma concentrations observed between 15 minutes and 2 hours. The mean steady-state Cmax was 23.3 ± 6.2 ng/mL and AUC0-12 averaged 78.0 ± 13.9 ng·h/mL.
Distribution: The protein binding of olopatadine was moderate at approximately 55% in human serum, and independent of drug concentration over the range of 0.1 to 1000 ng/mL. Olopatadine was bound predominately to human serum albumin.
Elimination: The plasma elimination half-life of olopatadine is 8 to 12 hours. Olopatadine is mainly eliminated through urinary excretion. Approximately 70% of a [14C] olopatadine hydrochloride oral dose was recovered in urine with 17% in the feces. Of the drug-related material recovered within the first 24 hours in the urine, 86% was unchanged olopatadine with the balance comprised of olopatadine N-oxide and N-desmethyl olopatadine.
Metabolism: Olopatadine is not extensively metabolized. Based on plasma metabolite profiles following oral administration of [14C] olopatadine, at least six minor metabolites circulate in human plasma. Olopatadine accounts for 77% of peak plasma total radioactivity and all metabolites amounted to <6% combined. Two of these have been identified as the olopatadine N-oxide and N-desmethyl olopatadine. In in vitro studies with cDNA-expressed human cytochrome P450 isoenzymes (CYP) and flavin-containing monooxygenases (FMO), N-desmethyl olopatadine (Ml) formation was catalyzed mainly by CYP3A4, while olopatadine N-oxide (M3) was primarily catalyzed by FMO1 and FMO3. Olopatadine at concentrations up to 33,900 ng/mL did not inhibit the in vitro metabolism of specific substrates for CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4. The potential for olopatadine and its metabolites to act as inducers of CYP enzymes has not been evaluated.
Special Population:
Patients with Hepatic Impairment: No specific pharmacokinetic study examining the effect of hepatic impairment was conducted. Since metabolism of olopatadine is a minor route of elimination, no adjustment of the dosing regimen of olopatadine hydrochloride nasal spray is warranted in patients with hepatic impairment.
Patients with Renal Impairment: The mean Cmax values for olopatadine following single intranasal doses were not markedly different between healthy subjects (18.1 ng/mL) and patients with mild, moderate and severe renal impairment (range 15.5 to 21.6 ng/mL). Mean plasma AUC0-12 was two-fold higher in patients with severe impairment (creatinine clearance <30 mL/min/1.73 m2). In these patients, peak steady-state plasma concentrations of olopatadine are approximately 10-fold lower than those observed after higher 20 mg oral doses, twice daily, which were well-tolerated. These findings indicate that no adjustment of the dosing regimen of olopatadine hydrochloride nasal spray is warranted in patients with renal impairment.
Male and Female Patients: The mean systemic exposure (Cmax and AUC0-12) in female SAR patients following multiple administration of olopatadine was 40% and 27% higher, respectively than those values observed in male SAR patients.
Racial or Ethnic Groups: The effects of race on olopatadine pharmacokinetics have not been adequately investigated.
Pediatric Patients 6 to 11 Years of Age:The systemic pharmacokinetics of olopatadine, olopatadine N-oxide and N-desmethyl olopatadine in patients 6 through 11 years of age were characterized using data from 42 pediatric patients administered olopatadine hydrochloride nasal spray, one spray per nostril twice daily for a minimum of 14 days. The mean Cmax (15.4 ± 7.3 ng/mL) of olopatadine was approximately 2-fold less than was comparable to that observed in adults (78.0 ± 13.9 ng·h/mL). The Cmax and AUC0-12 of olopatadine N-oxide were comparable to that observed in adults. The Cmax and AUC0-12 of N-desmethyl olopatadine are approximately 18% and 37% higher than that observed in adults, respectively.
Pediatric Patients 2 to 5 Years of Age: The systemic pharmacokinetics of olopatadine, olopatadine N-oxide, and N-desmethyl olopatadine were characterized using population pharmacokinetic methods applied to sparse data (approximately 5 samples per patient) obtained from 66 pediatric patients (2 to less than 6 years of age) administered one-half the recommended adult dose (1 spray per nostril) of olopatadine hydrochloride nasal spray twice daily for a minimum of 14 days. The mean Cmax and AUC0-12 of olopatadine were 13.4 ± 4.6 ng/mL and 75.0± 26.4 ng·hr/mL respectively. The mean Cmax and AUC0-12 of olopatadine N-oxide and N-desmethyl olopatadine were similar to that of patients 6 to 11 years of age.
Drug Interaction Studies
Drug interactions with inhibitors of liver enzymes are not anticipated because olopatadine is eliminated predominantly by renal excretion. Olopatadine did not inhibit the in vitro metabolism of specific substrates for CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4. Based on these data, drug interactions involving P450 inhibition are not expected. Due to the modest protein binding of olopatadine (55%), drug interactions through displacement from plasma proteins are also not expected.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Olopatadine demonstrated no tumorigenic potential in mice at oral doses up to 500 mg/kg/day (approximately 460 times the MRHDID on a mg/m2 basis) for 78 weeks or in rats at oral doses up to 200 mg/kg/day (approximately 370 times the MRHDID on a mg/m2 basis) for 104 weeks.
Mutagenesis
No mutagenic potential was observed when olopatadine was tested in an in vitro bacterial reverse mutation (Ames) test, an in vitro mammalian chromosome aberration assay or an in vivo mouse micronucleus test.
Impairment of Fertility
Olopatadine administered at an oral dose of 400 mg/kg/day, (approximately 730 times the MRHDID for adults on an mg/m2 basis) produced toxicity in male and female rats, and resulted in a decrease in the fertility index and reduced implantation rate. No effects on reproductive function were observed at 50 mg/kg/day (approximately 90 times the MRHDID on a mg/m2 basis).
14 CLINICAL STUDIES
Adult and Adolescent Patients 12 Years of Age and Older:
The efficacy and safety of olopatadine hydrochloride nasal spray were evaluated in three randomized, double blind, parallel group, multicenter, placebo (vehicle nasal spray)-controlled clinical trials of 2 weeks duration in adult and adolescent patients, 12 years of age and older with symptoms of seasonal allergic rhinitis. The three clinical trials were conducted in the United States and included 1,598 patients (556 males, and 1,042 females) 12 years of age and older. In these three trials 587 patients were treated with olopatadine hydrochloride nasal spray 0.6%, 418 patients were treated with olopatadine hydrochloride nasal spray 0.4%, and 593 patients were treated with vehicle nasal spray. Assessment of efficacy was based on patient recording of 4 individual nasal symptoms (nasal congestion, rhinorrhea, itchy nose, and sneezing) on a 0 to 3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) as reflective or instantaneous scores. Reflective scoring required patients to record symptom severity over the previous 12 hours; the instantaneous scoring required patients to record symptom severity at the time of recording. The primary efficacy endpoint was the difference from placebo in the percent change from baseline in the average of morning and evening reflective total nasal symptom score (rTNSS) averaged for the 2-week treatment period. In all 3 trials, patients treated with olopatadine hydrochloride nasal spray, two sprays per nostril, twice-daily, exhibited statistically significantly greater decreases in rTNSS compared to vehicle nasal spray. Results for the rTNSS from two representative trials are shown in Table 3.
Table 3: Mean Reflective Total Nasal Symptom Score (rTNSS) in Adult and Adolescent Patients with Seasonal Allergic Rhinitis
|
Treatment |
N |
Baseline |
Change from Baseline |
Difference from Placebo |
|||
|
Estimate |
95% CI |
p-value |
|||||
|
Study 1 |
Olopatadine Hydrochloride Nasal Spray 0.6% |
183 |
8.71 |
-3.63 |
-0.96 |
(-1.42, -0.51) |
<0.0001 |
|
Olopatadine Hydrochloride Nasal Spray 0.4% |
188 |
8.90 |
-3.38 |
-0.71 |
(-1.17, -0.26) |
0.0023 |
|
|
Vehicle Nasal Spray |
191 |
875 |
-2.67 | ||||
|
Study 2 |
Olopatadine Hydrochloride Nasal Spray 0.6% |
220 |
9.17 |
-2.90 |
-0.98 |
(-1.37, -0.59) |
<0.0001 |
|
Olopatadine Hydrochloride Nasal Spray 0.4% |
228 |
9.26 |
-2.63 |
-0.72 |
(-1.11, -0.33) |
0.0003 |
|
|
Vehicle Nasal Spray |
223 |
9.07 |
-1.92 | ||||
Abbreviation: CL, confidence interval.
Itchy eyes and watery eyes were evaluated as secondary endpoints but eye redness was not evaluated. In two of the studies, patients treated with olopatadine hydrochloride nasal spray had significantly greater decreases in reflective symptom scores for itchy eyes and watery eyes, compared to vehicle nasal spray.
In the 2-week seasonal allergy trials, onset of action was also evaluated by instantaneous TNSS assessments twice-daily after the first dose of study medication. In these trials, onset of action was seen after 1 day of dosing. Onset of action was evaluated in three environmental exposure unit studies with single doses of olopatadine hydrochloride nasal spray. In these studies, patients with seasonal allergic rhinitis were exposed to high levels of pollen in the environmental exposure unit and then treated with either olopatadine hydrochloride nasal spray or vehicle nasal spray, two sprays in each nostril, after which they self-reported their allergy symptoms hourly as instantaneous scores for the subsequent 12 hours. Olopatadine hydrochloride nasal spray 0.6% was found to have an onset of action of 30 minutes after dosing in the environmental exposure unit.
Pediatric Patients 6 to 11 Years of Age:
There were 3 clinical trials of 2 weeks duration with olopatadine nasal spray in patients 6 to 11 years of age with seasonal allergic rhinitis. Efficacy of olopatadine hydrochloride nasal spray was evaluated in 2 of the 3 trials. One of the 2 trials that showed efficacy was a randomized, double blind, parallel group, multicenter, placebo (vehicle nasal spray)-controlled clinical trial of 2 weeks duration including 1,188 children ages 6 to < 12 years with seasonal allergic rhinitis. Assessment of efficacy was based on patient/caregiver recording of 4 individual nasal symptoms (nasal congestion, rhinorrhea, itchy nose, and sneezing) on a 0 to 3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) as reflective or instantaneous scores. Reflective scoring captured symptom severity over the previous 12 hours; the instantaneous scoring captured symptom severity at the time of recording. The primary efficacy endpoint was the difference from placebo in the percent change from baseline in the average of patient/caregiver -reported morning and evening reflective total nasal symptom score (rTNSS) averaged for the 2- week treatment period. Patients treated with olopatadine hydrochloride nasal spray, 1 or 2 sprays per nostril twice daily, had statistically significantly greater decreases in rTNSS compared to vehicle nasal spray. Results for rTNSS are shown in Table 4.
Table 4: Mean Reflective Total Nasal Symptom Score (rTNSS) in Pediatric Patients 6 to 11 Years of Age with Seasonal Allergic Rhinitis
|
Treatment |
N |
Baseline |
Change from Baseline |
Difference from Placebo |
||
|
Estimate |
95% CI |
p-value |
||||
|
Olopatadine Hydrochloride Nasal Spray 0.6%, 1 spray per nostril twice daily |
294 |
8.99 |
-2.24 |
-0.55 |
(-0.90, -0.19) |
0.0015 |
|
Vehicle Nasal Spray, 1 spray per nostril twice daily |
294 |
9.09 |
-1.70 | |||
Abbreviation: CL, confidence interval.
Itchy eyes and watery eyes were evaluated as secondary endpoints in the same study but eye redness was not evaluated. Patients treated with olopatadine hydrochloride nasal spray had significantly greater decreases in reflective symptom scores for itchy eyes and watery eyes, compared to vehicle nasal spray.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Olopatadine Hydrochloride Nasal Spray, 665 mcg is supplied in a white plastic bottle with a metered-dose manual spray pump, a white nasal applicator, a white overcap and a safety clip in a box of 1 (NDC 50383-943-23). Each trade size bottle contains 30.5 g of clear, colorless liquid and will provide 240 metered sprays. After priming [see Dosage and Administration (2.3)], each spray delivers a fine mist containing 665 mcg of olopatadine hydrochloride in 100 microliters of formulation through the nozzle.
Net content 30.5 g, 240 sprays: NDC 50383-943-23
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Local Nasal Effects and Other Common Adverse Reactions
Inform patients that treatment with olopatadine hydrochloride nasal spray may lead to adverse reactions, which include epistaxis and nasal ulcerations [see Warnings and Precautions (5.1)]. Other common adverse reactions reported with use of olopatadine hydrochloride nasal spray include bitter taste, headache, and pharyngolaryngeal pain [see Adverse Reactions (6)].
Somnolence and Impaired Mental Alertness
Somnolence has been reported in some patients taking olopatadine hydrochloride nasal spray. Caution patients against engaging in hazardous occupations requiring complete mental alertness and motor coordination such as driving or operating machinery after administration of olopatadine hydrochloride nasal spray [see Warnings and Precautions (5.2)].
Concurrent Use of Alcohol and other Central Nervous System Depressants
Advise patients that concurrent use of olopatadine hydrochloride nasal spray with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur [see Warnings and Precautions (5.2)].
17.4 Keep Spray Out of Eyes
Inform patients to avoid spraying olopatadine hydrochloride nasal spray in their eyes [see Dosage and Administration (2.3)].
Distributed by:
Akorn Operating Company LLC
Gurnee, IL 60031
Rev.943:05 11/22