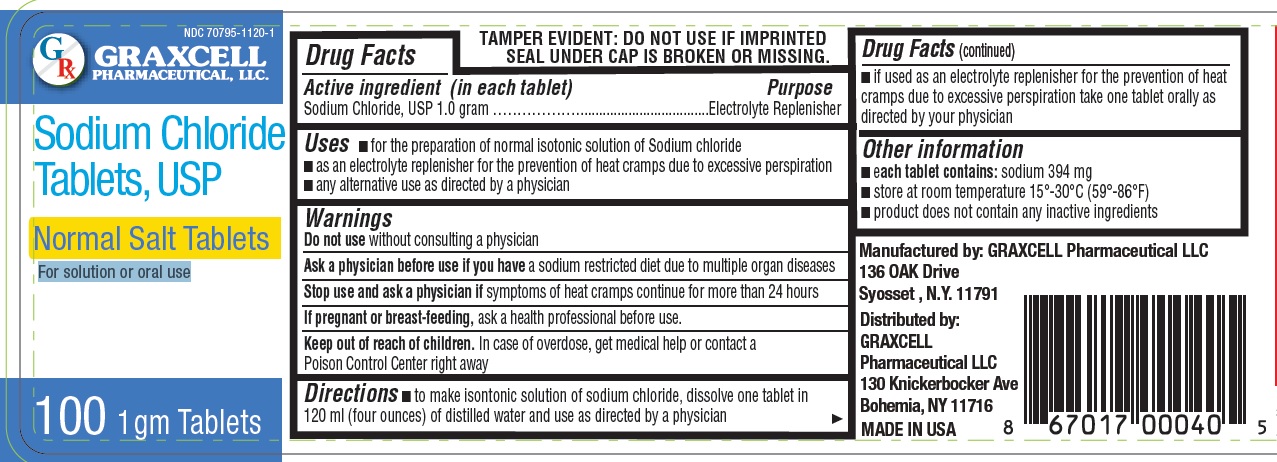
NORMAL SALT- sodium chloride tablet
GRAXCELL PHARMACEUTICAL, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each tablet)
Sodium Chloride, USP 1.0 gram
Purpose
Electrolyte Replenisher
Uses
- for the preparation of normal isotonic solution of Sodium chloride
- as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration
- any alternative use as directed by a physician
Warnings
Enter section text here
Do not use
without consulting a physician
Ask a physician before use
if you have a sodium restricted diet due to multiple organ diseases
Stop use and ask a physician
If symptoms of heat cramps continue for more than 24 hours
If pregnant or breast-feeding
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away
Directions
- to make isontonic solution of sodium chloride, dissolve one tablet in 120 ml (four ounces) of distilled water and use as directed by a physician
- if used as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration take one tablet orally as
directed by your physician
Other information
- each tablet contains: sodium 394 mg
- store at room temperature 15°-30°C (59°-86°F)
- product does not contain any inactive ingredients
INACTIVE INGREDIENTS
NONE