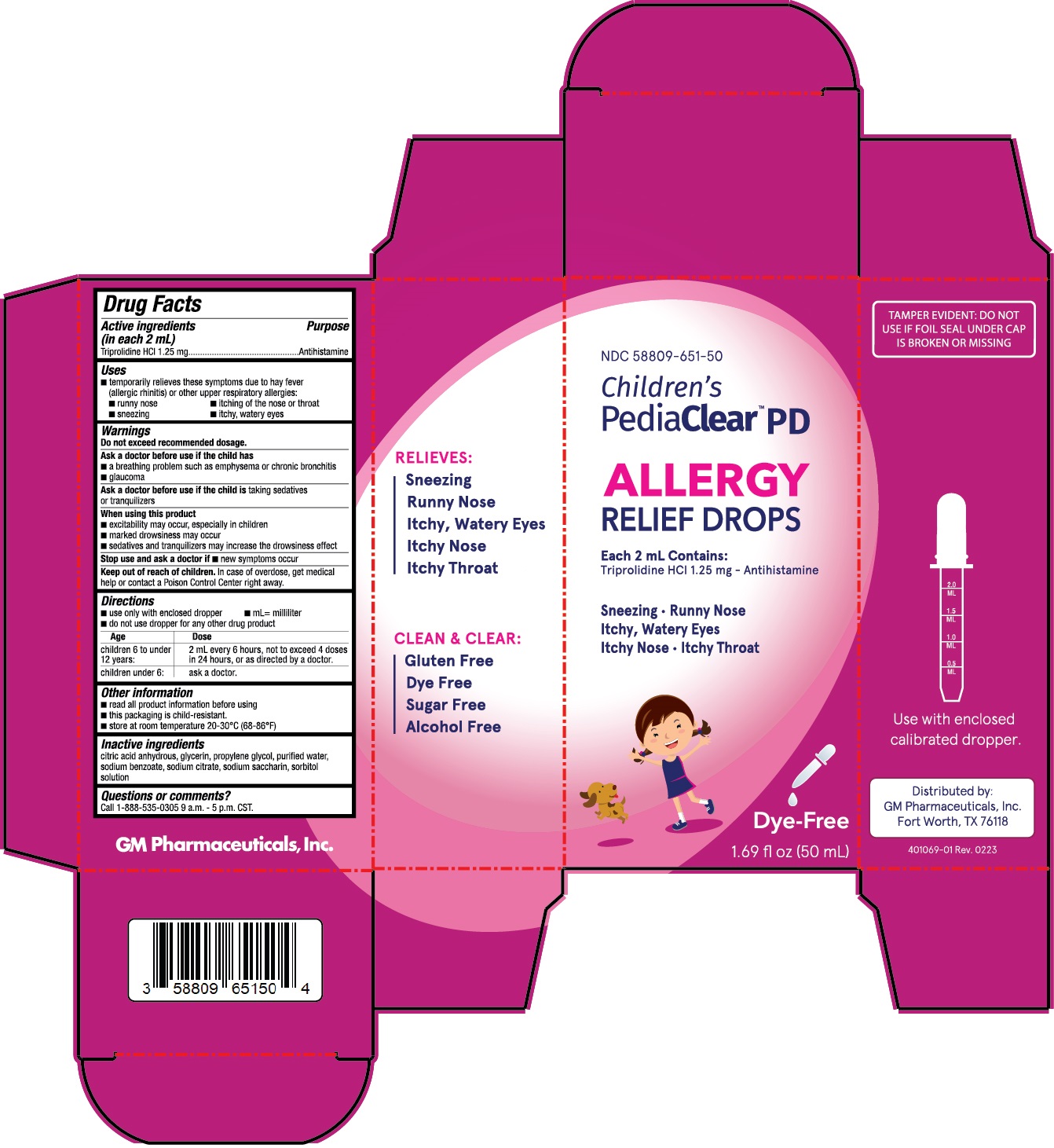
NDC 58809-651-50
Drug Facts
Active ingredients (in each 2 mL)
Triprolidine HCI 1.25 mg
Uses
■ temporarily relieves these symptoms due to hay fever
(allergic rhinitis) or other upper respiratory allergies:
■ runny nose ■ itching of the nose or throat
■ sneezing ■ itchy, watery eyes
Warnings
Do not exceed recommended dosage.
Ask a doctor before use if the child has
■ a breathing problem such as emphysema or chronic bronchitis
■ glaucoma
Ask a doctor before use if the child is
taking sedatives or tranquilizers
When using this product
■ excitability may occur, especially in children
■ marked drowsiness may occur
■ sedatives and tranquilizers may increase the drowsiness effect
Stop use and ask a doctor if
■ new symptoms occur
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
■ use only with enclosed dropper ■ mL= milliliter
■ do not use dropper for any other drug product
| Age | Dose |
| Children 6 to under 12 years: | 2 mL every 6 hours, not to exceed 4 doses in 24 hours, or as directed by a doctor. |
| Children under 6: | Ask a doctor. |
Other information
■ read all product information before using
■ this packaging is child-resistant.
■ store at room temperature 20-30°C (68-86°F)
Inactive ingredients
citric acid anhydrous, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sodium saccharin, sorbitol solution
Questions or comments?
Call 1-888-535-0305 9 a.m. - 5 p.m. CST.
PRINCIPAL DISPLAY PANEL
NDC 58809-651-50
Children’s PediaClear PD Drops
1.69 fl oz (50 mL)
Children’s PediaClear PD Drops Label

Children’s PediaClear PD Drops Drug Facts
Children’s PediaClear PD Drops Carton