DESCRIPTION
Dicyclomine hydrochloride is an antispasmodic and anticholinergic (antimuscarinic) agent. Dicyclomine hydrochloride occurs as a fine, white, crystalline, practically odorless powder with a bitter taste. It is soluble in water, freely soluble in alcohol and chloroform, and very slightly soluble in ether.
Chemically, it is [Bicyclohexyl]-1-carboxylic acid, 2-(diethyl-amino) ethyl ester, hydrochloride with the following structural formula:
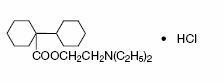
C19H35NO2•HCI 345.95
Each capsule, for oral administration, contains 10 mg of dicyclomine hydrochloride.
Each tablet, for oral administration, contains 20 mg of dicyclomine hydrochloride.
This product contains the following inactive ingredients: colloidal silicon dioxide (tablets only), corn starch (tablets only), D&C red #28 (capsules only), FD&C blue #1 (capsules only), FD&C blue #1 lake (tablets only), FD&C red #40 (capsules only), gelatin (capsules only), hypromellose (tablets only), lactose monohydrate (tablets only), magnesium stearate (capsules only), pregelatinized starch, silicon dioxide (capsules only), sodium lauryl sulfate (capsules only), sodium starch glycolate (tablets only), and stearic acid (tablets only).
CLINICAL PHARMACOLOGY
Dicyclomine relieves smooth muscle spasm of the gastrointestinal tract. Animal studies indicate that this action is achieved via a dual mechanism: (1) a specific anticholinergic effect (antimuscarinic) at the acetylcholine- receptor sites with approximately 1/8 the milligram potency of atropine (in vitro, guinea pig ileum); and (2) a direct effect upon smooth muscle (musculotropic) as evidenced by dicyclomine's antagonism of bradykinin- and histamine-induced spasms of the isolated guinea pig ileum. Atropine did not affect responses to these two agonists. In vivo studies in cats and dogs showed dicyclomine to be equally potent against acetylcholine (ACh)- or barium chloride (BaCI2)- induced intestinal spasm while atropine was at least 200 times more potent against effects of ACh than BaCI2. Tests for mydriatic effects in mice showed that dicyclomine was approximately 1/500 as potent as atropine; antisialogogue tests in rabbits showed dicyclomine to be 1/300 as potent as atropine.
In man, dicyclomine is rapidly absorbed after oral administration, reaching peak values within 60-90 minutes. The principal route of elimination is via the urine (79.5% of the dose). Excretion also occurs in the feces, but to a lesser extent (8.4%). Mean half-life of plasma elimination in one study was determined to be approximately 1.8 hours when plasma concentrations were measured for 9 hours after a single dose. In subsequent studies, plasma concentrations were followed for up to 24 hours after a single dose, showing a secondary phase of elimination with a somewhat longer half-life. Mean volume of distribution for a 20 mg oral dose is approximately 3.65 L/kg suggesting extensive distribution in tissues.
In controlled clinical trials involving over 100 patients who received drug, 82% of patients treated for functional bowel/irritable bowel syndrome with dicyclomine hydrochloride at initial doses of 160 mg daily (40 mg q.i.d.) demonstrated a favorable clinical response compared with 55% treated with placebo (p<.05). In these trials, most of the side effects were typically anticholinergic in nature (see table) and were reported by 61% of the patients.
| Dicyclomine | ||
| Hydrochloride | ||
| (40 mg q.i.d.) | Placebo | |
| Side Effect | % | % |
| Dry Mouth | 33 | 5 |
| Dizziness | 29 | 2 |
| Blurred Vision | 27 | 2 |
| Nausea | 14 | 6 |
| Light-headedness | 11 | 3 |
| Drowsiness | 9 | 1 |
| Weakness | 7 | 1 |
| Nervousness | 6 | 2 |
Nine percent (9%) of patients were discontinued from the drug because of one or more of these side effects (compared with 2% in the placebo group). In 41% of the patients with side effects, side effects disappeared or were tolerated at the 160 mg daily dose without reduction. A dose reduction from 160 mg daily to an average daily dose of 90 mg was required in 46% of the patients with side effects who then continued to experience a favorable clinical response; their side effects either disappeared or were tolerated. (See ADVERSE REACTIONS.)
CONTRAINDICATIONS
-
Obstructive uropathy
-
Obstructive disease of the gastrointestinal tract
-
Severe ulcerative colitis (See PRECAUTIONS)
-
Reflux esophagitis
-
Unstable cardiovascular status in acute hemorrhage
-
Glaucoma
-
Myasthenia gravis
-
Evidence of prior hypersensitivity to dicyclomine hydrochloride or other ingredients of these formulations
-
Infants less than 6 months of age (See WARNINGS and PRECAUTIONS: Information for Patients.)
-
Nursing Mothers (See WARNINGS and PRECAUTIONS: Information for Patients).
WARNINGS
In the presence of a high environmental temperature, heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). If symptoms occur, the drug should be discontinued and supportive measures instituted.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful.
Dicyclomine may produce drowsiness or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
Psychosis has been reported in sensitive individuals given anticholinergic drugs. CNS signs and symptoms include confusion, disorientation, short-term memory loss, hallucinations, dysarthria, ataxia, coma, euphoria, decreased anxiety, fatigue, insomnia, agitation and mannerisms, and inappropriate affect. These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the drug.
There are reports that administration of dicyclomine syrup to infants has been followed by serious respiratory symptoms (dyspnea, shortness of breath, breathlessness, respiratory collapse, apnea, asphyxia), seizures, syncope, pulse rate fluctuations, muscular hypotonia, and coma. Death has been reported. No causal relationship between these effects observed in infants and dicyclomine administration has been established. DICYCLOMINE IS CONTRAINDICATED IN INFANTS LESS THAN 6 MONTHS OF AGE AND IN NURSING MOTHERS. (See CONTRAINDICATIONS, and PRECAUTIONS: Nursing Mothers and Pediatric Use).
Safety and efficacy of dicyclomine hydrochloride in pediatric patients have not been established.
PRECAUTIONS
General
Use with caution in patients with:
-
Autonomic neuropathy
-
Hepatic or renal disease
-
Ulcerative colitis—large doses may suppress intestinal motility to the point of producing a paralytic ileus and the use of this drug may precipitate or aggravate the serious complication of toxic megacolon (see CONTRAINDICATIONS)
-
Hyperthyroidism
-
Hypertension
-
Coronary heart disease
-
Congestive heart failure
-
Cardiac tachyarrhythmia
-
Hiatal hernia (see CONTRAINDICATIONS: reflux esophagitis)
-
Known or suspected prostatic hypertrophy.
Investigate any tachycardia before administration of dicyclomine hydrochloride, since it may increase the heart rate.
With overdosage, a curare-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis).
Information For Patients
Dicyclomine may produce drowsiness or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or to perform hazardous work while taking this drug.
Dicyclomine is contraindicated in infants less than 6 months of age and in nursing mothers. (See CONTRAINDICATIONS, WARNINGS, and PRECAUTlONS: Nursing Mothers and Pediatric Use).
In the presence of a high environmental temperature, heat prostration can occur with drug use (fever and heat stroke due to decreased sweating).
If symptoms occur, the drug should be discontinued and a physician contacted.
Drug Interactions
The following agents may increase certain actions or side effects of anticholinergic drugs: amantadine, antiarrhythmic agents of Class I (e.g., quinidine), antihistamines, antipsychotic agents (e.g., phenothiazines), benzodiazepines, MAO inhibitors, narcotic analgesics (e.g., meperidine), nitrates and nitrites, sympathomimetic agents, tricyclic antidepressants, and other drugs having anticholinergic activity.
Anticholinergics antagonize the effects of antiglaucoma agents. Anticholinergic drugs in the presence of increased intraocular pressure may be hazardous when taken concurrently with agents such as corticosteroids. (See also CONTRAINDICATIONS.)
Anticholinergic agents may affect gastrointestinal absorption of various drugs, such as slowly dissolving dosage forms of digoxin; increased serum digoxin concentrations may result. Anticholinergic drugs may antagonize the effects of the drugs that alter gastrointestinal motility, such as metoclopramide. Because antacids may interfere with the absorption of anticholinergic agents, simultaneous use of these drugs should be avoided.
The inhibiting effects of anticholinergic drugs on gastric hydrochloric acid secretion are antagonized by agents used to treat achlorhydria and those used to test gastric secretion.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no known human data on long-term potential for carcinogenicity or mutagenicity.
Long-term studies in animals to determine carcinogenic potential are not known to have been conducted.
In studies in rats at doses of up to 100 mg/kg/day, dicyclomine produced no deleterious effects on breeding, conception, or parturition.
Teratogenic Effects
Pregnancy Category B.
Reproduction studies have been performed in rats and rabbits at doses up to 33 times the maximum recommended human dose based on 160 mg/day (3 mg/kg) and have revealed no evidence of impaired fertility or harm to the fetus due to dicyclomine. Epidemiologic studies in pregnant women with products containing dicyclomine hydrochloride (at doses up to 40 mg/day) have not shown that dicyclomine increases the risk of fetal abnormalities if administered during the first trimester of pregnancy. There are, however, no adequate and well-controlled studies in pregnant women at the recommended doses (80-160 mg/day). Because animal reproduction studies are not always predictive of human response, dicyclomine as indicated for functional bowel/irritable bowel syndrome should be used during pregnancy only if clearly needed.
Nursing Mothers
Since dicyclomine has been reported to be excreted in human milk, DICYCLOMINE HYDROCHLORIDE IS CONTRAINDICATED IN NURSING MOTHERS. (See CONTRAINDICATIONS, WARNINGS, PRECAUTIONS: Pediatric Use, and ADVERSE REACTIONS).
Pediatric Use
(See CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: Nursing Mothers.) DICYCLOMINE IS CONTRAINDICATED IN INFANTS LESS THAN 6 MONTHS OF AGE.
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
Controlled clinical trials have provided frequency information for reported adverse effects of dicyclomine hydrochloride listed in a decreasing order of frequency. (See CLINICAL PHARMACOLOGY.)
Not all of the following adverse reactions have been reported with dicyclomine hydrochloride. Adverse reactions are included here that have been reported for pharmacologically similar drugs with anticholinergic/antispasmodic action.
Gastrointestinal: dry mouth, nausea, vomiting, constipation, bloated feeling, abdominal pain, taste loss, anorexia
Central Nervous System: dizziness, light-headedness, tingling, headache, drowsiness, weakness, nervousness, numbness, mental confusion and/or excitement (especially in elderly persons), dyskinesia, lethargy, syncope, speech disturbance, insomnia
Ophthalmologic: blurred vision, diplopia, mydriasis, cycloplegia, increased ocular tension
Dermatological/Allergic: rash, urticaria, itching, and other dermal manifestations; severe allergic reaction or drug idiosyncrasies including anaphylaxis
Genitourinary: urinary hesitancy, urinary retention
Cardiovascular: tachycardia, palpitations
Respiratory: Dyspnea, apnea, asphyxia (see WARNINGS)
Other: decreased sweating, nasal stuffiness or congestion, sneezing, throat congestion, impotence, suppression of lactation (see PRECAUTlONS: Nursing Mothers)
DRUG ABUSE AND DEPENDENCE
Abuse of and/or dependence on dicyclomine for anticholinergic effects have been rarely reported.
OVERDOSAGE
Signs and Symptoms
The signs and symptoms of overdosage are headache; nausea; vomiting; blurred vision; dilated pupils; hot, dry skin; dizziness; dryness of the mouth; difficulty in swallowing; and CNS stimulation. A curare-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis).
A 37-year-old female reported numbness on the left side, cold fingertips, blurred vision, abdominal and flank pain, decreased appetite, dry mouth, and nervousness following ingestion of 320 mg daily (four 20 mg tablets QID) for four days. These events resolved after discontinuing the dicyclomine.
Oral LD50
The acute oral LD50 of the drug is 625 mg/kg in mice.
Minimum Human Lethal Dose/Maximum Human Dose Recorded
The amount of drug in a single dose that is ordinarily associated with symptoms of overdosage or that is Iikely to be life threatening, has not been defined. The maximum human oral dose recorded was 600 mg by mouth in a 10-month-old child and approximately 1500 mg in an adult, each of whom survived.
In three of the infants who died following administration of dicyclomine hydrochloride (see WARNINGS), the blood concentrations of drug were 200, 220, and 505 ng/mL, respectively.
DOSAGE AND ADMINISTRATION
DOSAGE MUST BE ADJUSTED TO INDIVIDUAL PATIENT NEEDS. (See CLINICAL PHARMACOLOGY.)
The only oral dose clearly shown to be effective is 160 mg per day (in 4 equally divided doses). Since this dose is associated with a significant incidence of side effects, it is prudent to begin with 80 mg per day (in 4 equally divided doses). Depending upon the patient's response during the first week of therapy, the dose should be increased to 160 mg per day unless side effects limit dosage escalation.
If efficacy is not achieved within 2 weeks or side effects require doses below 80 mg per day, the drug should be discontinued. Documented safety data are not available for doses above 80 mg daily for periods longer than 2 weeks.
The intramuscular dosage form is to be used temporarily when the patient cannot take oral medication. Intramuscular injection is about twice as bioavailable as oral dosage forms; consequently, the recommended intramuscular dose is 80 mg daily (in 4 equally divided doses).
Oral dicyclomine hydrochloride should be started as soon as possible and the intramuscular form should not be used for periods longer than 1 or 2 days.
HOW SUPPLIED
Dicyclomine Hydrochloride Capsules USP and Dicyclomine Hydrochloride Tablets USP are supplied as follows:
10 mg capsules: Clear Dark Blue cap/Clear Dark Blue body hard gelatin capsules, imprinted with white ink WATSON over 794 on cap and 10 mg on the body.
20 mg tablets: Blue, round, unscored, flat-faced, beveled-edge tablets, debossed WATSON and 795 on the periphery on one side and plain on the other side.
They are supplied by State of Florida DOH Central Pharmacy as follows:
| NDC | Strength | Quantity/Form | Color | Source Prod. Code |
| 53808-0233-1 | 10 mg | 30 Capsules in a Blister Pack | dark blue | 0591-0794 |
| 53808-0234-1 | 20 mg | 30 Tablets in a Blister Pack | BLUE | 0591-0795 |
Storage
Store at controlled room temperature 15°-30°C (59°-86°F).
Dispense in a well-closed container as defined in USP/NF.
Manufactured for:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Manufactured by:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 45215 USA
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States

