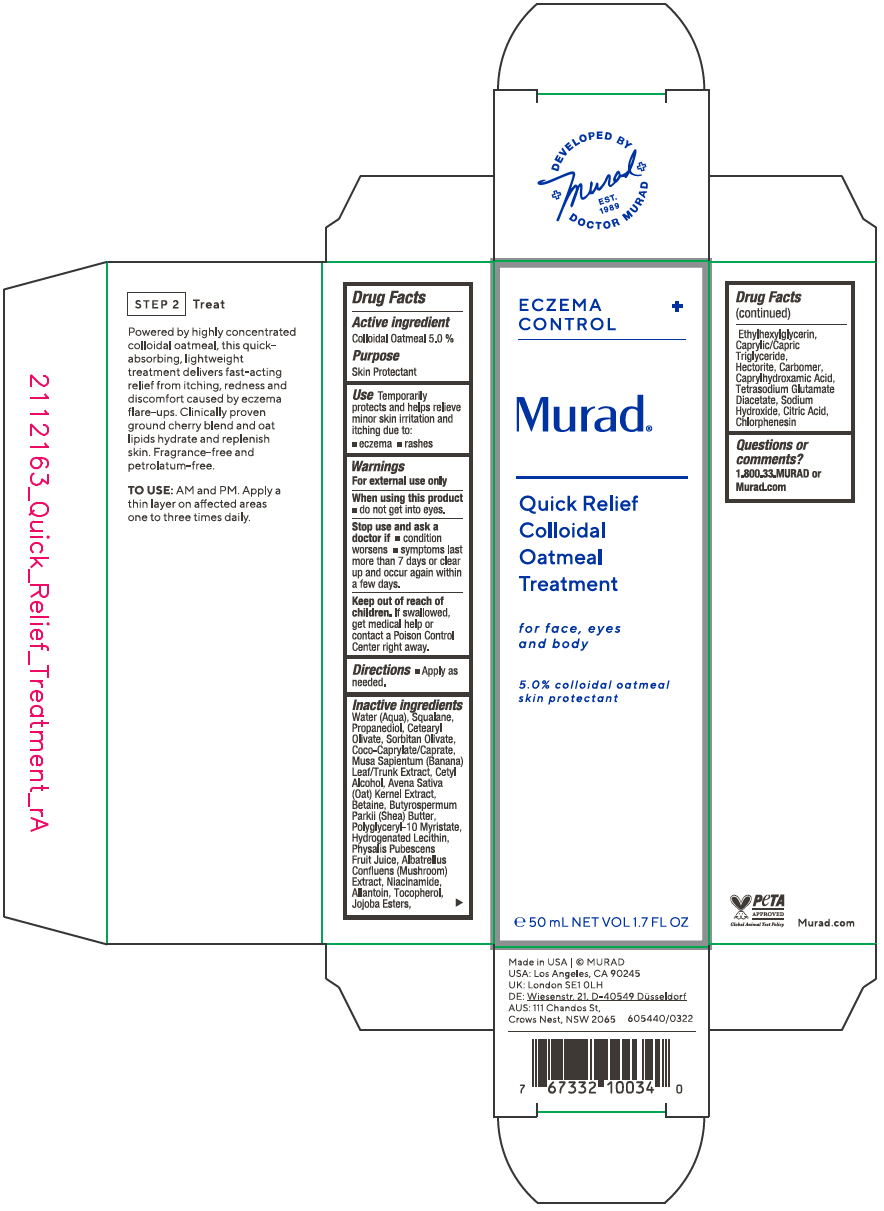
QUICK RELIEF COLLOIDAL OATMEAL TREATMENT FOR FACE, EYES AND BODY- colloidal oatmeal solution
Murad, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Colloidal Oatmeal 5.0 %
Use
Temporarily protects and helps relieve minor skin irritation and itching due to:
Warnings
For external use only
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
Water (Aqua), Squalane, Propanediol, Cetearyl Olivate, Sorbitan Olivate, Coco-Caprylate/Caprate, Musa Sapientum (Banana) Leaf/Trunk Extract, Cetyl Alcohol, Avena Sativa (Oat) Kernel Extract, Betaine, Butyrospermum Parkii (Shea) Butter, Polyglyceryl-10 Myristate, Hydrogenated Lecithin, Physalis Pubescens Fruit Juice, Albatrellus Confluens (Mushroom) Extract, Niacinamide, Allantoin, Tocopherol, Jojoba Esters, Ethylhexylglycerin, Caprylic/Capric Triglyceride, Hectorite, Carbomer, Caprylhydroxamic Acid, Tetrasodium Glutamate Diacetate, Sodium Hydroxide, Citric Acid, Chlorphenesin
Questions or comments?
1.800.33.MURAD or Murad.com
PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton
ECZEMA
CONTROL
+
Murad®
Quick Relief
Colloidal
Oatmeal
Treatment
for face, eyes
and body
5.0% colloidal oatmeal
skin protectant
℮ 50 mL NET VOL 1.7 FL OZ