LEGEND- hyaluronate sodium injection, solution
Merial, Inc.
----------
Legend®
(hyaluronate sodium)
Injectable Solution
DESCRIPTION:
LEGEND Injectable Solution is a clear, colorless solution of low viscosity. LEGEND Injectable Solution is pyrogen free, sterile and does not contain a preservative. It is administered by intravenous or intra-articular injection.
Hyaluronic acid, the conjugate acid of hyaluronate sodium, is extracted from the capsule of Streptococcus spp. and purified, resulting in a form which is essentially free of protein and nucleic acids.
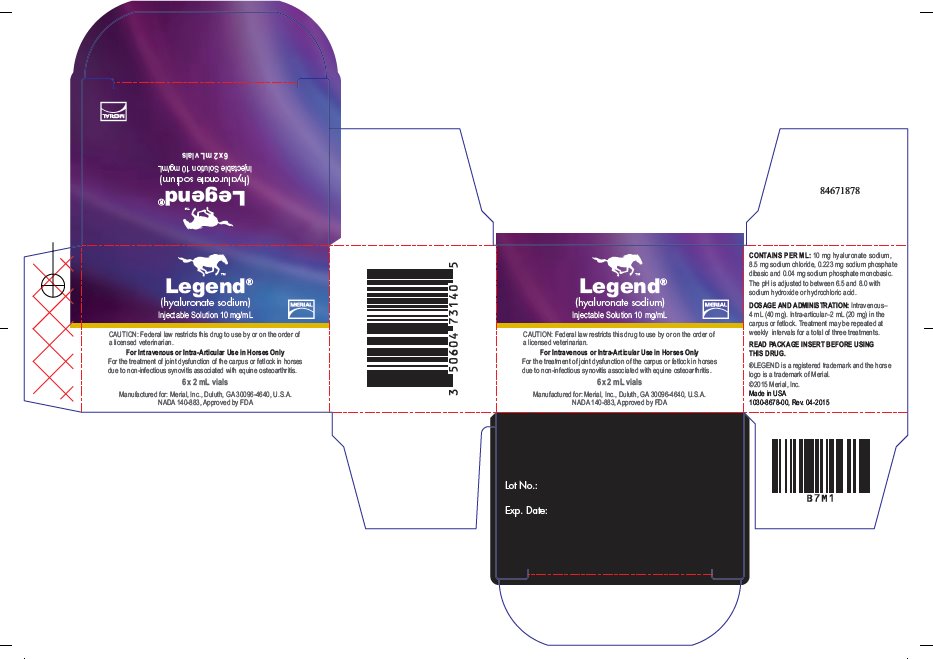
LEGEND Injectable Solution is supplied in 2 mL (20 mg) vials. Each mL contains 10 mg hyaluronate sodium, 8.5 mg sodium chloride, 0.223 mg sodium phosphate dibasic, and 0.04 mg sodium phosphate monobasic. The pH is adjusted to between 6.5 and 8.0 with sodium hydroxide or hydrochloric acid.
CHEMISTRY:
Hyaluronic acid, a glycosaminoglycan, can exist in the following forms depending upon the chemical environment in which it is found: as the acid, hyaluronic acid; as the sodium salt, sodium hyaluronate (hyaluronate sodium); or as the hyaluronate anion. These terms may be used interchangeably but in all cases, reference is made to the glycosaminoglycan composed of repeating subunits of D-glucuronic acid and N-acetyl-D-glucosamine linked together by glycosidic bonds. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulfate or any other glycosaminoglycan.
CLINICAL PHARMACOLOGY:
Hyaluronic acid is a naturally occurring substance present in connective tissue, skin, vitreous humour and the umbilical cord in all mammals. High concentrations of hyaluronic acid are also found in the synovial fluid. It also constitutes the major component of the capsule of certain microorganisms. The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals.
The actual mechanism of action for hyaluronate sodium in the healing of degenerative joint disease is not completely understood. One major function appears to be the regulation of normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity. Additionally, hyaluronate sodium exerts an anti-inflammatory action by inhibiting the movement of granulocytes and macrophages.1
Hyaluronate molecules are long chains which form a filter network interspersed with normal cellular fluids. It is widely accepted that injection directly into the joint pouch enhances the healing of inflamed synovium by restoring lubrication of the joint fluid. This further supplements the visco-elastic properties of normal joint fluid.
INDICATIONS:
LEGEND Injectable Solution is indicated in the treatment of joint dysfunction of the carpus or fetlock in horses due to non-infectious synovitis associated with equine osteoarthritis.
DOSAGE AND ADMINISTRATION:
Intravenously-4 mL (40 mg), Intra-articular- 2 mL (20 mg) in the carpus or fetlock. Treatment may be repeated at weekly intervals for a total of three treatments.
Strict aseptic technique should be observed when administering by intra-articular injection. As with any intra-articular procedure, proper injection site disinfection and animal restraint are important. Excess joint fluid should be aseptically removed prior to injection. Care should be taken to avoid scratching the cartilage surface with the tip of the injection needle. Diffuse swelling lasting 24 to 48 hours may result from movement of the needle while in the joint space.
For intravenous administration, use aseptic technique and inject slowly into the jugular vein.
Horses should be given stall rest after treatment before gradually resuming normal activity.
Discard any unused portion of the drug and the empty vial after opening.
PRECAUTIONS:
Radiographic evaluation should be carried out in cases of acute lameness to ensure that the joint is free from serious fractures. As with any intra-articular treatment, special precautions must be followed as to injection technique and sterility for prevention of possible swelling or infection. Intra-articular injections should not be made through skin that has been recently fired or blistered, or that has excessive scurf and counterirritant on it.
The safety of LEGEND Injectable Solution has not been evaluated in breeding stallions or in breeding, pregnant or lactating mares.
ADVERSE REACTIONS:
No local or systemic side effects were observed in the clinical field trials with either intravenous or intra-articular injections.
Post-Approval Experience: While all adverse reactions are not reported, the following adverse reactions are based on voluntary post-approval reporting:
Following intravenous use: Occasional depression, lethargy, and fever.
Following intra-articular use: lameness, joint effusion, joint or injection site swelling, and joint pain.
To report suspected adverse events, for technical assistance or to obtain a copy of the MSDS, contact Merial at 1-888-637-4251. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth/ProductSafetyInformation.
EFFECTIVENESS:
Forty-six horses with lameness in either the carpal or fetlock joints were treated intravenously or intra-articularly with LEGEND Injectable Solution in a well-controlled clinical study conducted at four locations. One, two or three injections were given based on clinical improvement. Overall clinical improvement was judged as excellent or good in 90% of the cases treated intravenously and 96% of those treated intra-articularly with LEGEND Injectable Solution.
ANIMAL SAFETY:
LEGEND Injectable Solution was administered to normal horses at one, three and five times the recommended intra-articular dosage of 20 mg and the intravenous dosage of 40 mg. Treatments were given once weekly for nine consecutive weeks (three times the maximum duration). No systemic clinical signs were observed nor were there any adverse effects upon hematology or clinical chemistry parameters. A transient, slight to mild post-injection swelling of the joint capsule occurred in some of the animals treated intra-articularly with LEGEND Injectable Solution as it did in the saline treated control horses. No gross or histological lesions were observed in the soft tissues or the surface areas of the treated joint.
HOW SUPPLIED:
LEGEND Injectable Solution is supplied in a carton of six 2 mL (20 mg) bottles.
NADA 140-883, Approved by FDA
Manufactured for: Merial, Inc., Duluth, GA 30096-4640, U.S.A.
REFERENCE:
1Swanstrom, O.G. 1978. Hyaluronate (hyaluronic acid) and its use, Proc. American Assoc. Equine Pract., 24th annual convention, pp. 345-348.
®LEGEND is a registered trademark and the horse logo is a trademark of Merial.
©2015 Merial, Inc. All rights reserved.
Made in USA
1050-8681-01, Rev. 05-2015
84671851
PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton
Legend®
(hyaluronate sodium)
Injectable Solution 10 mg/mL
MERIAL
CAUTION: Federal law restricts this drug to use by or on the order of
a licensed veterinarian.
For Intravenous or Intra-Articular Use in Horses Only
For the treatment of joint dysfunction of the carpus or fetlock in horses
due to non-infectious synovitis associated with equine osteoarthritis.
6 x 2 mL vials
Manufactured for: Merial, Inc., Duluth, GA 30096-4640, U.S.A.
NADA 140-883, Approved by FDA
| LEGEND
hyaluronate sodium injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merial, Inc. (799641006) |