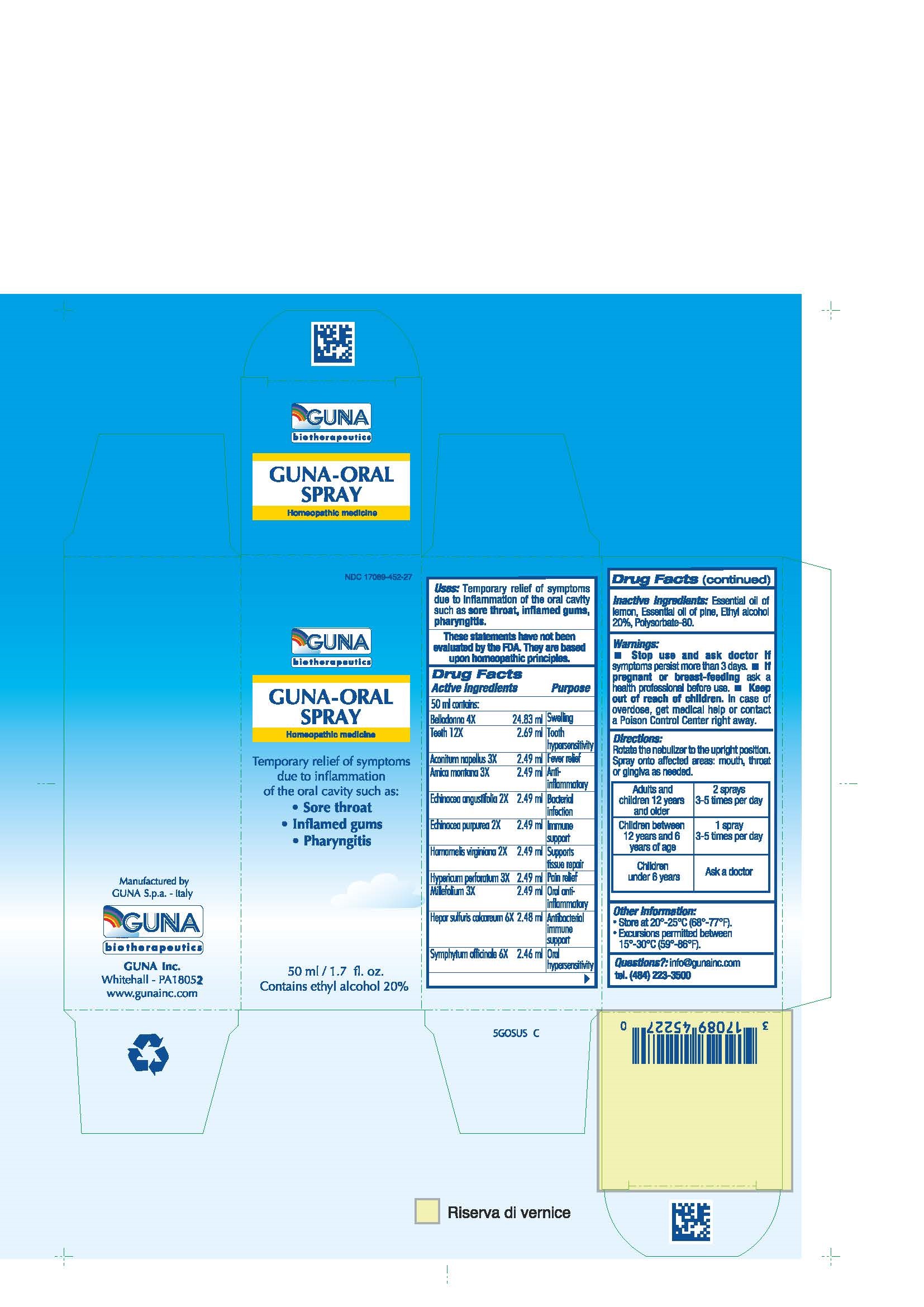
ACTIVE INGREDIENTS/PURPOSE
ACONITUM NAPELLUS 3X ANTI-INFLAMMATORY
ARNICA MONTANA 3X FEVER RELIEF
BELLADONNA 4X SWELLING
ECHINACEA ANGUSTIFOLIA 2X BACTERIAL INFECTION
ECHINACEA PURPUREA 2X IMMUNE SUPPORT
HAMAMELIS VIRGINIANA 2X SUPPORTS TISSUE REPAIR
HEPAR SULFURIS CALCAREUM 6X ANTIBACTERIAL IMMUNE SUPPORT
HYPERICUM PERFORATUM 3X PAIN RELIEF
MILLEFOLIUM 3X ORAL ANTI- INFLAMMATORY
SYMPHYTUM OFFICINALE 6X ORAL HYPERSENSITIVITY
TEETH 12X TOOTH HYPERSENSITIVITY
USES
Temporary relief of symptoms due to inflammation of the oral cavity such as:
- Sore throat
- Inflamed gums
- Pharyngitis
WARNINGS
- Stop use and ask doctor if symptoms persist more than 3 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 20%
DIRECTIONS
Adults and children 12 years and older 2 sprays, 3-5 times per day
Children between 12 years and 6 years of age 1 spray t, 3-5 times per day
Children under 6 years ask a doctor
Directions: Rotate the nebulizer to the upright position. Spray onto affected areas: mouth, throat or gingiva as needed.