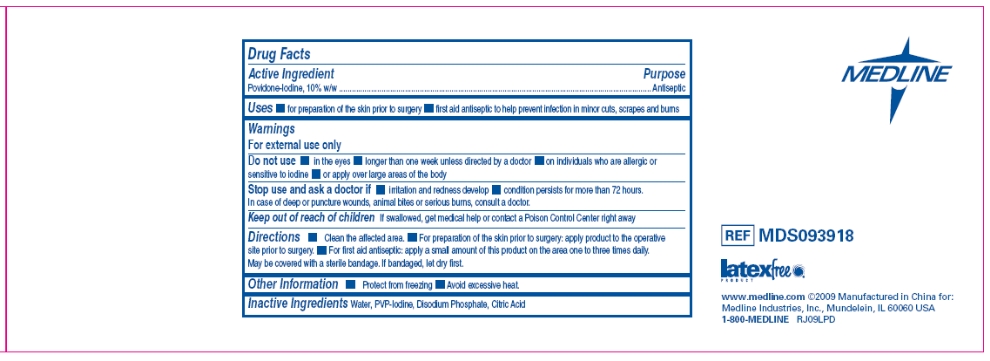
Uses
- Healthcare antiseptic for preparation of the skin prior to surgery
- First aid antiseptic to help prevent infection in minor cuts, scrapes and burns
Warnings
For external use only.
Do not use
- in the eyes
- longer than 1 week unless directed by a doctor
- on individuals who are allergic or sensitive to iodine
- or apply over large areas of the body
Directions
Clean the affected area.
- For preparation of the skin prior to surgery: apply product to the operative site prior to surgery
- For first aid antiseptic: apply a small amount of this product on the area 1 to 3 times daily. May be covered with a sterile bandage. If bandaged, let dry first.
Principal Display Panel - Swabstick Singles pouch

NDC53329-945-09
Medline
Antiseptic
Povidone-Iodine, USP
Swabsticks, Singles
MDS093901
latex free
For professional and hospital use.
Contents: 1 Swabstick
Principal Display Panel - Swabstick Singles box

NDC: 53329-945-29
Medline
Antiseptic
Povidone-Iodine, USP
Swabsticks, Singles
MDS093901
Discard appropriately after single use.
Protect from Freezing. Avoid excessive heat. For external use only.
latex free
Contents: 50 Each
Principal Display Panel - Swabstick Triples box

NDC 53329-946-75
Medline
Antiseptic
Povidone-Iodine, USP
Swabsticks, Triples
MDS093902
Discard appropriately after single use.
Protect from freezing. Avoid excessive heat. For external use only.
latex free
Contents: 25 Pouches
Principal Display Panel - Prep Pads, Medium box
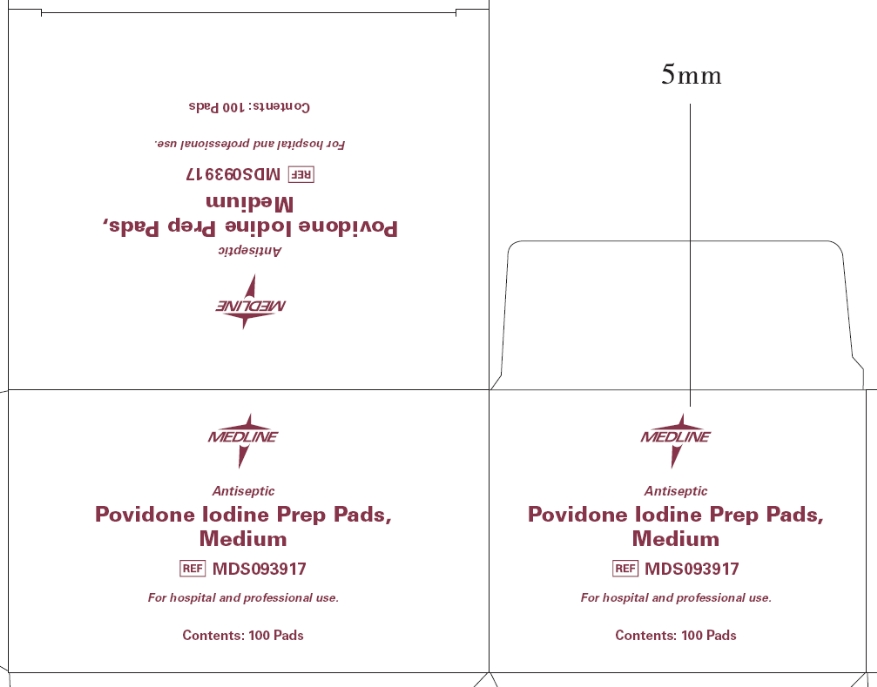
Medline
Antiseptic
Povidone Iodine Prep Pads, Medium
MDS093917
For hospital and professional use.
Contents: 100 Pads
Principal Display Panel - Prep Pad Bulk case

Povidone Iodine
Prep PADS
Medium Bulk
MDS093918
Contents: 3,000 Each Per Case
Principal Display Panel - Prep Solution bottle

NDC: 53329-939-13
MEDLINE
PVP Prep Solution
Topical Antiseptic
Povidone-Iodine 10%
MDS093940
latexfree
www.medline.com
Manufactured for: Medline Industries, Inc.
Mundelein, IL 60060 USA Made in USA
To Order Call: 1-800-MEDLINE
RB11CTR
2 FL OZ (59 mL)