FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Limited Population: Pretomanid Tablet is indicated, as part of a combination regimen with bedaquiline and linezolid for the treatment of adults with pulmonary tuberculosis (TB) resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug OR adults with pulmonary TB resistant to isoniazid and rifampin, who are treatment-intolerant or nonresponsive to standard therapy. Approval of this indication is based on limited clinical safety and efficacy data. This drug is indicated for use in a limited and specific population of patients.
Limitations of Use:
- •
- Take Pretomanid Tablets are not indicated in patients with:
- o
- Drug-sensitive (DS) TB
- o
- Latent infection due to Mycobacterium tuberculosis.
- o
- Extra-pulmonary infection due to Mycobacterium tuberculosis.
- o
- TB resistant to isoniazid and rifampin who are responsive to standard therapy and not treatment-intolerant
- o
- TB with known resistance to any component of the combination
- •
- Safety and effectiveness of Pretomanid Tablets have not been established for its use in combination with drugs other than bedaquiline and linezolid as part of the recommended dosing regimen [see Dosage and Administration (2.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- •
- Pretomanid Tablets must be used only in combination with bedaquiline and linezolid as part of the recommended dosing regimen [see Dosage and Administration (2.2)].
- •
- Emphasize the need for compliance with the full course of therapy to patients [see Patient Counseling Information (17)].
- •
- Administer the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid by directly observed therapy (DOT).
2.2 Recommended Dosage
Pretomanid Tablets must be administered in combination with bedaquiline and linezolid. The recommended dosage and duration for bedaquiline and linezolid when used in the combination regimen with Pretomanid Tablet are as follows:
- •
- Pretomanid Tablet 200 mg orally (1 tablet of 200 mg), once daily, for 26 weeks. Swallow Pretomanid Tablets whole with water.
- •
- Bedaquiline 400 mg orally once daily for 2 weeks followed by 200 mg 3 times per week, with at least 48 hours between doses, for 24 weeks for a total of 26 weeks.
- •
- Linezolid starting at 1,200 mg orally per day for 26 weeks, with dose adjustments to 600 mg daily and further reduction to 300 mg daily or interruption of dosing as necessary for known linezolid adverse reactions of myelosuppression, peripheral neuropathy, and optic neuropathy [see Dosage and Administration (2.4) and Warnings and Precautions (5.3, 5.4)].
- •
- Take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid with food [see Clinical Pharmacology (12.3)].
- •
- If the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid is interrupted by a healthcare provider for safety reasons, missed doses can be made up at the end of the treatment; doses of linezolid alone missed due to linezolid adverse reactions should not be made up.
- •
- Dosing of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can be extended beyond 26 weeks, if necessary [see Clinical Studies (14)].
2.3 Assessments Prior to Initiating the Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid
- •
- Assess for symptoms and signs of liver disease (such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, and hepatomegaly). Obtain laboratory tests (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and bilirubin) [see Warnings and Precautions (5.2)].
- •
- Obtain complete blood count [see Warnings and Precautions (5.3)]. Obtain serum potassium, calcium, and magnesium and correct if abnormal [see Warnings and Precautions (5.5)]. Obtain an ECG before initiation of treatment [see Warnings and Precautions (5.5)].
2.4 Discontinuation of Dosing
If either bedaquiline or Pretomanid Tablets are discontinued, the entire combination regimen should also be discontinued.
If linezolid is permanently discontinued during the initial four consecutive weeks of treatment, bedaquiline and Pretomanid Tablets should also be discontinued. If linezolid is discontinued after the initial four weeks of consecutive treatment, continue administering bedaquiline and Pretomanid Tablets [see Dosage and Administration (2.2)].
3 DOSAGE FORMS AND STRENGTHS
Pretomanid Tablets, 200 mg, are white to off-white oval tablets debossed with M on one side and P200 on the other side.
4 CONTRAINDICATIONS
Pretomanid Tablets used in the combination regimen with bedaquiline and linezolid are contraindicated in patients for whom bedaquiline and/or linezolid are contraindicated. Refer to the bedaquiline and linezolid prescribing information.
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with the Combination Treatment Regimen
Pretomanid Tablet is indicated for use as part of a regimen in combination with bedaquiline and linezolid. Refer to the prescribing information for bedaquiline and linezolid for additional risk information. Warnings and Precautions related to bedaquiline and linezolid also apply to their use in the combination regimen with Pretomanid Tablets.
5.2 Hepatotoxicity
Hepatic adverse reactions were reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)]. Avoid alcohol and hepatotoxic agents, including herbal supplements and drugs other than bedaquiline and linezolid [see Indications and Usage (1)] while on Pretomanid Tablets, especially in patients with impaired hepatic function.
Monitor symptoms and signs (such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, and hepatomegaly) and laboratory tests (ALT, AST, alkaline phosphatase, and bilirubin) at a minimum at baseline, at two weeks, and then monthly while on treatment and as needed. If evidence of new or worsening liver dysfunction occurs, test for viral hepatitides and discontinue other hepatotoxic medications. Interrupt treatment with the entire regimen if:
- •
- Aminotransferase elevations are accompanied by total bilirubin elevation greater than 2 times the upper limit of normal.
- •
- Aminotransferase elevations are greater than 8 times the upper limit of normal.
- •
- Aminotransferase elevations are greater than 5 times the upper limit of normal and persist beyond 2 weeks.
5.3 Myelosuppression
Myelosuppression (including anemia, leukopenia, thrombocytopenia, and pancytopenia) was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Myelosuppression is a known adverse reaction of linezolid. Anemia can be life threatening [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)]. When linezolid dosing, as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, was reduced, interrupted, or discontinued, the observed hematologic abnormalities were reversible. Complete blood counts should be monitored at a minimum at baseline, at two weeks, and then monthly in patients receiving linezolid as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, and decreasing or interrupting linezolid dosing should be considered in patients who develop or have worsening myelosuppression [see Dosage and Administration (2.2)].
5.4 Peripheral and Optic Neuropathy
Peripheral neuropathy and optic neuropathy were reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)]. Neuropathy is a known adverse reaction of long-term linezolid use. Neuropathy associated with linezolid is generally reversible or improved with appropriate monitoring and interruption, dose reduction, or discontinuation of linezolid dosing. Monitor visual function in all patients receiving the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid; if a patient experiences symptoms of visual impairment, interrupt linezolid dosing and obtain prompt ophthalmologic evaluation.
5.5 QT Prolongation
QT prolongation was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1), Adverse Reactions (6.1), and Clinical Pharmacology (12.2)]. QT prolongation is a known adverse reaction of bedaquiline. Obtain an ECG before initiation of treatment, and at least 2, 12, and 24 weeks after starting treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Obtain serum potassium, calcium, and magnesium at baseline and correct if abnormal. Monitor these electrolytes if QT prolongation is detected [see Adverse Reactions (6.1)].
The following may increase the risk for QT prolongation when patients are receiving bedaquiline as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid: a history of Torsade de Pointes, congenital long QT syndrome, ongoing hypothyroidism, ongoing bradyarrhythmia, uncompensated heart failure, or serum calcium, magnesium, or potassium levels below the lower limits of normal. If necessary, bedaquiline treatment initiation could be considered in these patients after a favorable benefit-risk assessment and with frequent ECG monitoring.
Discontinue the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if the patient develops clinically significant ventricular arrhythmia or a QTcF interval of greater than 500 ms (confirmed by repeat ECG). If syncope occurs, obtain an ECG to detect QT prolongation.
5.6 Drug Interactions
CYP3A4 Inducers
Pretomanid may be in part metabolized by CYP3A4 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Avoid co‑administration of strong or moderate CYP3A4 inducers, such as rifampin or efavirenz, during treatment with pretomanid.
5.7 Reproductive Effects
Pretomanid caused testicular atrophy and impaired fertility in male rats. Advise patients of reproductive toxicities seen in animal studies and that the potential effects on human male fertility have not been adequately evaluated [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
5.8 Lactic Acidosis
Lactic acidosis was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)]. Lactic acidosis is a known adverse reaction of linezolid. Patients who develop recurrent nausea or vomiting should receive immediate medical evaluation, including evaluation of bicarbonate and lactic acid levels, and interruption of linezolid or the entire combination regimen of Pretomanid Tablets, bedaquiline, and linezolid should be considered.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed here and elsewhere in the labeling:
- •
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- •
- Myelosuppression [see Warnings and Precautions (5.3)]
- •
- Peripheral and Optic Neuropathy [see Warnings and Precautions (5.4)]
- •
- QT Prolongation [see Warnings and Precautions (5.5)]
- •
- Reproductive Effects [see Warnings and Precautions (5.7)]
- •
- Lactic Acidosis [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
When Pretomanid Tablets are administered in combination with bedaquiline and linezolid, refer to the prescribing information for the respective drugs for a description of the adverse reactions associated with their use.
More than 1691 subjects, at least 1348 patients with tuberculosis and 343 healthy volunteers, have been exposed to Pretomanid Tablets, either alone or as part of a combination therapy in 24 trials.
The registrational trial, Trial 1 (NCT02333799), was a single-arm, open-label trial conducted in three sites in South Africa in which adult patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug or pulmonary TB resistant to isoniazid and rifampin, who were treatment intolerant or non-responsive to standard therapy received the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 6 months (extendable to 9 months) with 24 months of follow up. One hundred and nine subjects were treated; 76% were black, and 23% were of mixed race. Their ages ranged from 17 years to 60 years (mean 36 years), and all patients were from South Africa. Fifty-six (51%) patients were HIV-positive. There were 8 deaths. Six patients died while receiving treatment; all surviving patients, excluding one patient who withdrew consent, completed treatment. Two patients died during follow-up at Day 369 and Day 486, respectively.
Common Adverse Reactions Reported in Trial 1
Table 1 summarizes the incidence of select adverse reactions occurring in ≥5% of patients in Trial 1.
| Pretomanid Tablets, Bedaquiline and Linezolid Combination Regimen
(N = 109) |
|
|---|---|
| Adverse Reactions | All Grades n (%) |
|
|
|
Peripheral neuropathy* |
88 (81) |
|
Acne* |
42 (39) |
|
Anemia* |
40 (37) |
|
Nausea |
40 (37) |
|
Vomiting |
37 (34) |
|
Musculoskeletal Pain* |
32 (29) |
|
Headache |
30 (28) |
|
Transaminases increased* |
30 (28) |
|
Dyspepsia |
26 (24) |
|
Decreased appetite |
24 (22) |
|
Rash* |
23 (21) |
|
Pruritus* |
22 (20) |
|
Abdominal pain* |
21 (19) |
|
Pleuritic pain |
21 (19) |
|
Gamma-glutamyltransferase increased |
19 (17) |
|
Lower respiratory tract infection* |
16 (15) |
|
Hyperamylasemia* |
15 (14) |
|
Hemoptysis |
14 (13) |
|
Cough* |
13 (12) |
|
Visual impairment* |
13 (12) |
|
Hypoglycemia |
12 (11) |
|
Abnormal loss of weight |
11 (10) |
|
Diarrhea |
11 (10) |
|
Constipation |
9 (8) |
|
Gastritis |
9 (8) |
|
Neutropenia* |
9 (8) |
|
Dry skin |
8 (7) |
|
Hypertension* |
8 (7) |
|
Electrocardiogram QT prolonged |
6 (6) |
|
Hyperlipasemia* |
6 (6) |
|
Insomnia |
6 (6) |
|
Thrombocytopenia* |
6 (6) |
The following select adverse reactions were reported in patients receiving the combination regimen of Pretomanid Tablets, bedaquiline and linezolid at a rate of less than 5% in Trail 1:
Gastrointestinal Disorders: pancreatitis, dysgeusia
Laboratory Investigations: blood creatine phosphokinase increase, blood creatinine increase, blood alkaline phosphatase increase
Blood and Lymphatic System Disorders: leukopenia
Metabolism and Nutrition Disorders: hypomagnesemia, hyperglycemia, hypokalemia, hyperkalemia, hyponatremia
Nervous System Disorders: dizziness, seizure
Laboratory Abnormalities Reported in Trail 1
Table 2 summarizes select laboratory abnormalities.
| Parameter Multiples of Upper Limit of Normal (x ULN) | Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid (N = 109) n (%) |
|---|---|
| ULN = upper limit of normal | |
|
Transaminases and Bilirubin | |
|
Alanine Aminotransferase (ALT) | |
|
>3 and ≤ 5 X ULN |
6 (6) |
|
>5 and ≤ 8 X ULN |
5 (5) |
|
>8 X ULN |
1 (1) |
|
Aspartate Aminotransferase (AST) | |
|
>3 and ≤ 5 X ULN |
7 (6) |
|
>5 and ≤ 8 X ULN |
2 (2) |
|
>8 X ULN |
1 (1) |
|
Total Bilirubin | |
|
>1 X ULN and ≤ 2 X ULN |
6 (6) |
|
>2 X ULN |
2 (2) |
|
Hematology | |
|
Hemoglobin | |
|
≤7.9 g/dL |
6 (6) |
|
Neutrophils Absolute Count | |
|
≤749/mm3 |
5 (5) |
|
Platelets | |
|
≤49,999/mm3 |
2 (2) |
|
Serum Chemistry | |
|
Lipase | |
|
> 2 X ULN |
5 (5) |
In Trail 1, 28% of patients experienced increased transaminases. Except for one patient who died due to pneumonia and sepsis, all patients who experienced increased transaminases were able to continue therapy and complete the full course of treatment.
Myelosuppression is a known adverse reaction of linezolid. The most common hematopoietic cytopenia was anemia (37%). The majority of cytopenias began after 2 weeks of treatment. Three patients experienced cytopenias that were considered serious: neutropenia in 1 patient and anemia in 2 patients. All 3 serious adverse reactions resulted in interruption of linezolid or all components of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, and all resolved.
Peripheral and Optic Neuropathy
Peripheral neuropathy is a known adverse reaction of linezolid. In Trial 1, peripheral neuropathy was reported in 81% of patients. Most of these adverse reactions (64%) occurred after 8 weeks of treatment and resulted in dosing interruption, dose reduction, or discontinuation of linezolid. Severe, moderate, and mild peripheral neuropathy occurred in 22%, 32%, and 26% of patients, respectively. No adverse reaction related to peripheral neuropathy led to a discontinuation of the entire study regimen.
Optic neuropathy is a known adverse reaction of linezolid. Two patients (2%) in Trial 1 developed optic neuropathy after 16 weeks of treatment. Both were serious, confirmed on retinal examination as optic neuropathy/neuritis, and resulted in discontinuation of linezolid; both adverse reactions resolved.
Overall, patients administered a linezolid dose of 600 mg twice daily had a similar safety profile to those administered a dose of 1,200 mg once daily.
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Pretomanid
CYP3A4 Inducers
Co-administration of pretomanid with rifampin and efavirenz resulted in a decrease in pretomanid plasma concentrations [see Clinical Pharmacology (12.3)]. Avoid co-administration of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid with rifampin, efavirenz, or other strong or moderate CYP3A4 inducers. Refer to the prescribing information for bedaquiline for additional information about drug interactions with CYP3A4.
Lopinavir/ritonavir
Co-administration of pretomanid with lopinavir/ritonavir did not affect the plasma concentrations of pretomanid [see Clinical Pharmacology (12.3)]. Lopinavir/ritonavir can be co-administered with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
7.2 Effect of Pretomanid on Other Drugs
Midazolam
Co-administration of pretomanid with the CYP3A4 substrate, midazolam, resulted in no clinically significant effect on the pharmacokinetics of midazolam or its major metabolite, 1-hydroxy-midazolam [see Clinical Pharmacology (12.3)]. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can be administered with CYP3A4 substrate drugs.
Organic Anion Transporter-3 (OAT3), BCRP, OATP1B3 and P-gp Substrates
The effect of co-administration of pretomanid on the pharmacokinetics of OAT3 substrates in humans is unknown. However, in vitro studies indicate that pretomanid significantly inhibits the OAT3 drug transporter [see Clinical Pharmacology (12.3)], which could result in increased concentrations of OAT3 substrate drugs clinically and may increase the risk of adverse reactions associated with these drugs.
If pretomanid is co-administered with OAT3 substrate drugs (e.g., methotrexate, indomethacin, ciprofloxacin), increase monitoring for OAT3 substrate drug-related adverse reactions and consider dosage reduction for OAT3 substrate drugs, if needed. Refer to the prescribing information of the co-administered drug for dosage reduction information.
In vitro studies cannot exclude the possibility that pretomanid is an inhibitor of BCRP, OATP1B3 and P-gp [see Clinical Pharmacology (12.3)]. No clinical studies have been performed to investigate these interactions. Therefore, it cannot be excluded that co-administration of pretomanid with sensitive OATP1B3 substrates (e.g., valsartan, statins), BCRP substrates (e.g., rosuvastatin, prazosin, glyburide, sulfasalazine) and P-gp substrates (e.g., digoxin, dabigatran etexilate, verapamil) may increase their exposure. If pretomanid is co-administered with substrates of OATP1B3, BCRP, or P-gp, increased monitoring for drug-related adverse reactions to the co-administered medicinal product should be performed.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies or available data on pretomanid use in pregnant women to inform any drug-associated risks. There are risks associated with active tuberculosis during pregnancy (see Clinical Considerations). When Pretomanid Tablets are administered in combination with bedaquiline and linezolid, the pregnancy information for bedaquiline and linezolid also applies to this combination regimen. Refer to the bedaquiline and linezolid prescribing information for more information on bedaquiline and linezolid associated risks of use during pregnancy. In animal reproduction studies, there was increased post-implantation loss in the presence of maternal toxicity (reduced bodyweight and feed consumption) with oral administration of pretomanid during organogenesis in rats at doses about 4 times the exposure at the recommended dose in humans. There were no adverse embryo fetal effects in rats or rabbits dosed with oral pretomanid during organogenesis at doses up to approximately 2 times the exposure in humans.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Animal Data
In animal reproduction studies, pregnant rats were dosed orally with pretomanid at 10, 30, and 100 mg/kg/day during organogenesis (gestational Days 7 through 17). Rats showed increased post-implantation loss in the presence of maternal toxicity (including reduced body weight and feed consumption) at 100 mg/kg/day, approximately 4 times the exposure in humans for a 200 mg dose on an AUC basis. There were no adverse embryofetal effects in rats dosed with oral pretomanid during organogenesis at doses up to approximately 2 times the exposure in humans. Pregnant rabbits were dosed orally with pretomanid during organogenesis (gestational Days 7 through 19) at 10, 30, and 60 mg/kg/day. No evidence of adverse developmental outcomes was observed when oral doses of pretomanid were administered to dams during organogenesis (gestational Days 7 to 19) at doses up to 60 mg/kg/day (approximately 2 times the exposure in humans for a 200 mg dose on an AUC basis).
In a pre- and postnatal development study, there were no adverse developmental effects in pups of pregnant rats orally dosed with up to 20 mg/kg/day from gestational Day 6 through lactation Day 20. Pups of pregnant females dosed at 60 mg/kg/day (about 2 times the exposure for the 200 mg dose) had lower body weights and a slight delay in the age at which the air-drop righting reflex developed. These effects occurred at a maternally toxic dose (based on maternal weight loss and reduced food consumption).
8.2 Lactation
Risk Summary
There is no information regarding the presence of pretomanid in human milk, or its effects on milk production or the breastfed infant. Pretomanid was detected in rat milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for adverse reactions in nursing infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Pretomanid Tablets and any potential adverse effects on the breastfed infant from Pretomanid Tablets or from the underlying maternal condition. When Pretomanid Tablets are administered in combination with bedaquiline and linezolid, information on lactation for bedaquiline and linezolid also applies to this combination regimen. Refer to the bedaquiline and linezolid prescribing information for more information on their use during lactation.
Data
Animal Data
In a pre- and postnatal development study in rats treated with pretomanid at doses 0.5 and 2 times the human exposure for a 200 mg dose (AUC) from gestational day 7 through lactation day 20, concentrations in milk on lactation day 14 were 1.4 and 1.6 times higher than the maximum concentration observed in maternal plasma, respectively. The concentration of pretomanid in rat milk does not necessarily predict the concentration of pretomanid in human milk.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Reduced fertility and/or testicular toxicity were observed in male rats and mice treated with oral pretomanid. These effects were associated with hormonal changes including decreased serum inhibin B and increased serum follicle stimulating hormone and luteinizing hormone in rodents [see Nonclinical Toxicology (13.1)].
Reduced fertility and testicular toxicity cannot be definitively ruled out in male human subjects at this time.
8.4 Pediatric Use
Safety and effectiveness of Pretomanid Tablets in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
10 OVERDOSAGE
There is no experience with the treatment of acute overdose with pretomanid. Take general measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) in case of deliberate or accidental overdose.
11 DESCRIPTION
Pretomanid is an oral nitroimidazooxazine antimycobacterial drug.
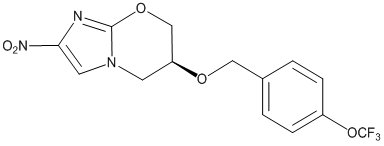
Pretomanid is a white to off-white to yellow-colored powder. The chemical name for pretomanid is (6S)-2-Nitro-6-{[4-(trifluoromethoxy)phenyl]methoxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine. The molecular formula for pretomanid is C14H12F3N3O5, and the molecular weight is 359.26. The structural formula of pretomanid is as follows:
Each Pretomanid Tablet contains 200 mg of pretomanid. The inactive ingredients are colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, and sodium starch glycolate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pretomanid is a nitroimidazooxazine antimycobacterial drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
A randomized, double-blind, placebo- and positive-controlled (moxifloxacin 400 mg), crossover, thorough QT study of pretomanid was performed in 74 healthy adult subjects. At 400 mg (2 times the approved recommended dosage) and 1,000 mg (5 times the approved recommended dosage) single doses of pretomanid, no significant QT prolongation effect was detected.
In Trial 1, patients received the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 6 months. No patient had QTcF intervals greater than 480 msec, and 1 subject had a post-baseline increase of QTcF of greater than 60 msec.
12.3 Pharmacokinetics
Pretomanid AUC and Cmax were approximately dose proportional over a range of single oral doses from 50 mg (0.25 times the approved recommended dosage) to 200 mg (approved recommended dosage); at single doses greater than 200 mg and up to 1,000 mg (5 times the approved recommended dosage), AUC and Cmax increased in a less than dose proportional manner. Steady-state pretomanid plasma concentrations were achieved approximately 4 to 6 days following multiple dose administration of 200 mg, and the accumulation ratio was approximately 2. Pharmacokinetic parameters following single and multiple 200 mg doses of pretomanid in healthy adult subjects are summarized in Table 3.
| ND - Not Determined. | |||
|
PK Parameter |
Single Dose 200 mg; Fasted |
Single Dose 200 mg; Fed |
Steady State 200 mg QD; Fasted |
|
Cmax (µg/mL) |
1.1 (0.2) |
2.0 (0.3) |
1.7 (0.3) |
|
AUCt (µg•hr/mL) |
*28.1 (8.0) |
*51.6 (10.1) |
†30.2 (3.7) |
|
AUCinf (µg•hr/mL) |
28.8 (8.3) |
53.0 (10.6) |
ND |
|
‡Tmax (hr) |
4.0 (2.0, 6.0) |
5.0 (3.0, 8.1) |
4.5 (2.0, 8.0) |
|
Vd/F (L) |
180 (51.3) |
97.0 (17.2) |
ND |
|
CL/F (L/hr) |
7.6 (2.5) |
3.9 (0.8) |
ND |
|
t½ (hr) |
16.9 (3.1) |
17.4 (2.8) |
16.0 (1.6) |
Absorption
Effect of Food
Administration of an oral tablet dose of pretomanid with a high-fat, high-calorie meal (approximately 150, 250, and 500 to 600 calories from protein, carbohydrate, and fat, respectively) increased mean Cmax by 76% and mean AUCinf by 88% as compared with the fasted state (see also Table 3 above).
Elimination
See Table 3 above for estimates of apparent oral clearance and half-life of pretomanid.
Specific Populations
No clinically significant differences in the pharmacokinetics of pretomanid were observed based on sex, body weight, race (Black, White, or other), pulmonary TB status (resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug OR resistant to isoniazid and rifampin and treatment intolerant or non-responsive to standard therapy), or HIV status. The effect of renal or hepatic impairment on the pharmacokinetics of pretomanid is unknown.
Drug Interaction Studies
Clinical Studies
Efavirenz: Co-administration of 200 mg QD of pretomanid with efavirenz 600 mg QD for 7 days resulted in a decrease of pretomanid mean AUC by 35% and Cmax by 28%. Mean AUC and Cmax of efavirenz were not affected when given with pretomanid.
Lopinavir/ritonavir: Co-administration of 200 mg QD pretomanid with lopinavir/ritonavir 400/100 mg BID for 7 days resulted in a decrease of pretomanid mean AUC by 17% and Cmax by 13%. Mean AUC and Cmax of lopinavir were decreased by 14% and 17%, respectively, when given with pretomanid.
Rifampin: Co-administration of 200 mg QD pretomanid with rifampin 600 mg QD for 7 days resulted in a decrease of pretomanid mean AUC by 66% and Cmax by 53%.
Midazolam: Co-administration of 400 mg (twice the approved recommended dosage) QD pretomanid for 14 days and a single 2 mg oral dose of midazolam on Day 14 resulted in a decrease in midazolam mean AUC by 15% and Cmax by 16%, and an increase in 1-hydroxy midazolam mean AUC by 14% and Cmax by 5%.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically
Cytochrome P450 (CYP) Enzymes: CYP3A4 plays a role in the metabolism of pretomanid, i.e., up to 20%. Pretomanid is not a substrate of CYP2C9, CYP2C19, and CYP2D6. Pretomanid is not an inhibitor of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 at clinically relevant concentrations based on in vitro studies. Pretomanid is not an inducer of CYP3A4.
Transporter Systems: Pretomanid is an inhibitor of the OAT3 transporter in vitro, which could result in increased concentrations of OAT3 substrate medicinal products clinically and may increase the risk of adverse reactions associated with these medicines. No clinical drug-drug interaction studies have been conducted with OAT3 substrates [see Drug Interactions (7.2)].
In vitro studies cannot exclude the possibility that pretomanid is an inhibitor of BCRP, OATP1B3, and P-gp transporters. The effect of co-administration of pretomanid on the pharmacokinetics of BCRP, OATP1B3 and P-gp substrates in humans is unknown [see Drug Interactions (7.2)].
In vitro studies indicated that pretomanid does not inhibit human OAT1, OCT1, OCT2, OAT1B1, BSEP, MATE1, and/or MATE2-K mediated transport at clinically relevant concentrations of pretomanid. Pretomanid is not a substrate of OAT1, OAT3, OCT2, OAT1B1, OATP1B3, MATE1, MATE2-K, BCRP, and/or P-gp transporters.
12.4 Microbiology
Mechanism of Action
Pretomanid Tablet is a nitroimidazooxazine antimycobacterial drug. Pretomanid kills actively replicating M. tuberculosis by inhibiting mycolic acid biosynthesis, thereby blocking cell wall production. Under anaerobic conditions, against non-replicating bacteria, pretomanid acts as a respiratory poison following nitric oxide release. All of these activities require nitro-reduction of pretomanid within the mycobacterial cell by the deazaflavin-dependent nitroreductase, Ddn, which is dependent on the reduced form of the cofactor F420. Reduction of F420 is accomplished by the F420-dependent glucose-6-phosphate dehydrogenase, Fgd1.
Resistance
Mutations in five M. tuberculosis genes (ddn, fgd1, fbiA, fbiB, and fbiC) have been associated with pretomanid resistance. The products of these genes are involved in bioreductive activation of pretomanid within the bacterial cell. Not all isolates with increased minimum inhibitory concentrations (MICs) have mutations in these genes, suggesting the existence of at least one other mechanism of resistance. The in vitro frequency of resistance development to pretomanid ranged from 10-7 to 10-5 at 2 to 6 times the pretomanid MICs. Cross-resistance of pretomanid with other compounds in the same class has been observed.
Antimicrobial Activity
Pretomanid has demonstrated in vitro activity against the M. tuberculosis complex. Pretomanid has also demonstrated anti-M. tuberculosis activity in animal models of tuberculosis [see Indications and Usage (1)].
In murine tuberculosis models, the 3-drug combination of pretomanid, bedaquiline, and linezolid reduced bacterial counts in the lungs to a greater extent and resulted in fewer relapses at 2 and 3 months post-therapy compared to 2-drug combinations of pretomanid, bedaquiline, and linezolid.
In Trial 1, the pretomanid MIC was determined using the Mycobacterial Growth Indicator Tube (MGIT). The baseline pretomanid MIC for M. tuberculosis isolates in the trial ranged from 0.06 to 1 mcg/mL.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No mutagenic or clastogenic effects were detected in conventional genotoxicity studies with pretomanid. A circulating metabolite of pretomanid, M50, was mutagenic in a bacterial reverse mutation assay. No carcinogenic potential was revealed in a 6-month study in transgenic mice where this metabolite was produced. In a 2-year study in rats, there was no evidence of carcinogenic risk.
Mutagenesis
No mutagenic or clastogenic effects were detected in both an in vitro bacterial reverse mutation assay and an in vitro mammalian chromosome aberrations assay using a Chinese hamster ovary cell line. Pretomanid was negative for clastogenicity in a mouse bone marrow micronucleus assay.
A metabolite of pretomanid was mutagenic in a bacterial reverse mutation assay. This metabolite represents approximately 6% of the human exposure (AUC) to pretomanid at the MRHD.
Fertility
In a fertility and general reproduction study in rats, male rats treated orally with pretomanid for 13 to 14 weeks had reduced fertility at 30 mg/kg/day and complete infertility at 100 mg/kg/day (approximately 1 and 2‑times the human exposure for a 200 mg dose, respectively). At 100 mg/kg/day, males had testicular atrophy including hypospermia in the epididymal tubules and focal epithelial hyperplasia of the epididymal tubular epithelium. Following a 10-week treatment-free period, these effects were partially reversed in male rats given pretomanid at 30 mg/kg/day but not at 100 mg/kg/day. These effects were associated with increased serum follicle-stimulating hormone and decreased serum inhibin B concentrations. There were no effects of pretomanid in male rats treated for 13 weeks at 10 mg/kg/day (approximately half of the human exposure for a 200 mg dose). Pretomanid did not affect mating behavior in female rats given oral pretomanid at 100 mg/kg/day for two weeks (approximately twice the human exposure).
Testicular toxicity was present in male mice treated orally for 13 weeks at 20 mg/kg/day [approximately equal to the human exposure (AUC) for a 200 mg dose]. There were no adverse testicular effects observed in mice given pretomanid at 6 mg/kg/day (0.2‑times the human exposure for a 200 mg dose).
Testicular toxicity was observed in male rats following 7 or 14 days of dosing with oral pretomanid at 100 mg/kg/day (approximately 2-times the human exposure for a 200 mg dose). The effects were partially reversible during a 6-month post treatment recovery period in rats treated with pretomanid for 7 days, but not 14 days.
In a 3-month study, decreased sperm motility and total sperm count, and increased abnormal sperm ratio were noted in sexually mature monkeys given ≥150 mg/kg/day (approximately 3 times the human exposure for a 200 mg dose).
13.2 Animal Toxicology and/or Pharmacology
Cataracts were observed in rats treated with pretomanid at doses of 300 mg/kg/day for 13 weeks or 100 mg/kg/day for 26 weeks. There were no cataracts observed in rats given oral pretomanid at 30 mg/kg/day (approximately 2 times the human exposure for a 200 mg dose) for 26 weeks.
In monkeys given oral pretomanid at 450 mg/kg/day for 4 weeks and 300 mg/kg/day for 12 more weeks, cataracts were not present at the end of dosing but developed during the 13‑week post treatment recovery period. In a subsequent study, cataracts were not observed following 13 weeks treatment with up to 300 mg/kg/day oral pretomanid or during the 20-week post treatment recovery period. Further, no cataracts were observed in monkeys given oral pretomanid at 100 mg/kg/day for 39 weeks with a 12-week post treatment recovery. This is approximately 1- to 2-times the human exposure for a 200 mg dose (AUC).
14 CLINICAL STUDIES
Trial 1 (NCT02333799) was an open-label trial conducted in three centers in South Africa in patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug (Population 1), or pulmonary TB resistant to isoniazid and rifampin, who were treatment intolerant or non-responsive to standard therapy (Population 2). Fifty-six (51%) patients were HIV-positive. The patients received a combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 6 months (extended to 9 months in 2 patients) with 24 months of follow-up; linezolid starting dose was either 600 mg twice daily or 1200 mg once daily. One hundred seven of the 109 patients enrolled were assessable for the primary efficacy analyses with two patients remaining in follow up for the primary outcome assessment.
Treatment failure was defined as the incidence of bacteriologic failure (reinfection – culture conversion to positive status with a different M. tuberculosis strain), bacteriological relapse (culture conversion to positive status with the same M. tuberculosis strain), or clinical failure (an unfavorable status at, or before, end of treatment (EOT) or failing to attain culture negativity, or if the patient was withdrawn at or before EOT for clinical reasons including retreatment or changing treatment) through follow-up until 6 months after the EOT. Results are presented in Table 4. Of the 107 patients assessed, outcomes were classified as success for 95 (89%) patients and failure for 12 (11%) patients. The success rate significantly exceeded the historical success rates for TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug based on a literature review. The outcomes were similar in both HIV negative and HIV positive patients.
| Population 1 = TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug; Population 2 = TB resistant to isoniazid and rifampin, who were treatment-intolerant or nonresponsive to standard therapy | ||||
|
||||
|
Outcome |
Total |
Population 1 |
Population 2 |
|
|
Total assessable |
107 |
71 |
36 |
|
|
Success |
Success (culture negative status at 6 months post treatment) |
95 (89%) |
63 (89%) |
32 (89%) |
|
Failure |
Death |
7 |
6 |
1 |
|
Relapse post treatment |
2 |
1* |
1 |
|
|
Withdrawal, loss to follow-up, or contaminated cultures |
3 |
1 |
2 |
|
|
Total Failure |
12 (11%) |
8 (11%) |
4 (11%) |
|
16 HOW SUPPLIED/STORAGE AND HANDLING
Pretomanid Tablet 200 mg is packaged in either white, round, high-density polyethylene bottles with polypropylene child-resistant closure or child-resistant blister packages comprised of a polyvinylchloride film with foil and paper backing.
Pretomanid Tablet 200 mg is a white to off-white, oval-shaped tablet debossed with M on one side and P200 on the other side.
|
NDC Number |
Size |
|
49502-476-26 |
Bottle of 26 |
|
49502-476-14 |
Unit dose blister pack of 14 (1 strip of 14 tablets) |
|
49502-476-72 |
Bottle of 182 |
Store below 30 °C (86 °F).
Dispense only in original container and keep container tightly closed.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Important Information on Co-administration of Pretomanid Tablets in Combination with Bedaquiline and Linezolid
- •
- Inform the patient or caregiver that Pretomanid Tablets administered as a combination regimen with bedaquiline and linezolid would be useful only in adult patients with TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug or TB resistant to isoniazid and rifampin, who are treatment-intolerant or nonresponsive to standard therapy. This regimen is not indicated for treatment in patients with latent infection or extra-pulmonary infection due to M. tuberculosis, drug-sensitive TB, TB resistant to isoniazid and rifampin who are responsive to standard therapy and not treatment-intolerant, or who have TB with known resistance to any component of the regimen (Pretomanid Tablets, bedaquiline, or linezolid).
- •
- Instruct the patient or caregiver that the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid must be administered by directly observed therapy (DOT).
- •
- Inform patients of safety concerns associated with linezolid and bedaquiline and advise the patient or their caregiver to read the Medication Guide for bedaquiline.
Adverse Reactions
Advise patients that the following serious adverse reactions can occur with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid: liver enzyme abnormalities, myelosuppression including anemia, peripheral and optic neuropathy, and cardiac rhythm abnormalities.
Peripheral and Optic Neuropathy
Advise patients to promptly inform their physician if they experience changes in vision during linezolid therapy. Monitor visual function in all patients receiving linezolid. Counsel patients to obtain prompt ophthalmological evaluation if the patient experiences symptoms of visual impairment.
Additional serious adverse reactions can occur with the use of linezolid, including serotonin syndrome, lactic acidosis, and convulsions. Refer to the prescribing information for linezolid for additional counseling information for these serious adverse reactions.
Interruption of Linezolid Dosing to Manage Linezolid Adverse Reactions
Counsel patients that linezolid dosing may be modified or interrupted during the therapy to manage the known linezolid adverse reactions of myelosuppression, peripheral neuropathy, and optic neuropathy.
Compliance with Treatment
Inform patients that Pretomanid Tablets must be taken as part of a combination regimen with bedaquiline and linezolid. Compliance with the full course of therapy must be emphasized. Advise patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed for the full prescribed duration of dosing. Skipping doses other than as directed by a physician or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that their Mycobacterium may develop resistance and the disease will not be treatable by the regimen or other antibacterial drugs in the future.
Administration Instructions
Inform patients to take the regimen with food. Doses of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid missed for safety reasons can be made up at the end of treatment; doses of linezolid alone missed due to linezolid adverse reactions should not be made up. If bedaquiline and/or Pretomanid Tablets are permanently discontinued, the entire combination regimen of Pretomanid Tablets, bedaquiline, and linezolid should be discontinued.
Use in Patients with Hepatic or Renal Impairment
Advise patients to inform their physician if they have a history of liver or kidney problems. The safety and effectiveness of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid in populations with hepatic or renal impairment have not been established.
Use with Alcohol and Other Medications
Advise patients to discuss with their physician the other medications they are taking and other medical conditions before starting treatment with Pretomanid Tablets.
Advise patients to abstain from alcohol, hepatotoxic medications, and herbal products.
Storage Instructions Advise patients to store Pretomanid Tablets, bedaquiline, and linezolid at room temperature below 86°F (30°C).
Manufactured by:
Mylan Laboratories Limited,
Hyderabad, 500 096, India
Manufactured for:
Mylan Specialty L.P.,
Morgantown, WV 26505 U.S.A.
Under license from TB Alliance.
MS:MXI:PRET:R3
MEDICATION GUIDE
|
Pretomanid Tablets (Pre-TOH-mah-nid) Limited Population |
|||
|
What is the most important information I should know about the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
Pretomanid Tablets are for use only as part of a combination antibiotic regimen that includes Pretomanid Tablets, bedaquiline, and linezolid.
Treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause serious side effects. See “What are the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?”
Read the Medication Guide that comes with bedaquiline. Ask your healthcare provider for information about linezolid. The serious side effects that can happen when taking bedaquiline and linezolid can also happen when taken in the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. |
|||
|
What is the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
Pretomanid Tablets are a prescription medicine used as part of a combination regimen with bedaquiline and linezolid. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid includes three prescription antibiotics that are used together in adults to treat tuberculosis (TB) of the lungs that is resistant to other classes of antibiotics (isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibiotic) or in adults who cannot tolerate or do not respond to treatment for TB that is resistant to two specific antibiotics (isoniazid and rifampin).
Pretomanid Tablets are not for use in people who have:
It is not known if Pretomanid Tablets are safe and effective for use except in combination with bedaquiline and linezolid as part of the recommended dosing regimen.
It is not known if Pretomanid Tablets are safe and effective in children. Pretomanid Tablets were approved by FDA using the Limited Population pathway. This means FDA has approved this drug for a limited and specific patient population, and studies on the drug may have only answered focused questions about its safety and effectiveness. |
|||
|
Do not take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if:
|
|||
|
Before you take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. You should not take herbal products or medicines that can harm your liver during treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid regimen may affect how other medicines work, and other medicines may affect how the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid works.
Especially tell your healthcare provider if you take:
|
|||
|
How should I take the combination regimen of Pretomanid Tablets, bedaquiline and linezolid?
Do not stop taking Pretomanid Tablets, bedaquiline, or linezolid without first talking to your healthcare provider. |
|||
|
What should I avoid when taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
|
|||
|
What are the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid? The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid may cause serious side effects including:
|
|||
|
|
||
|
|||
|
|
||
|
|||
|
|
||
The most common side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid include: |
|||
|
|
|
|
|
These are not all the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
How should I store Pretomanid Tablets?
Keep Pretomanid Tablets and all medicines out of the reach of children. |
|||
|
General information about the safe and effective use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Pretomanid Tablets, bedaquiline, or linezolid for a condition for which it was not prescribed. Do not give Pretomanid Tablets, bedaquiline, or linezolid to other people, even if they have the same symptoms that you have. This may harm them. You can ask your pharmacist or healthcare provider for information about Pretomanid Tablets, bedaquiline, and linezolid that is written for health professionals. |
|||
|
What are the ingredients in the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid? Pretomanid Tablets active ingredient: pretomanid
Pretomanid Tablets inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, and sodium starch glycolate
The ingredients for bedaquiline can be found in the Medication Guide for bedaquiline. The ingredients for linezolid can be found in the information about linezolid that is written for health professionals.
Manufactured by: Manufactured for: Under license from TB Alliance.
For more information call 1-877-446-3679. |
|||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 12/2022
MS:MXI:MG:PRET:R3
PRINCIPAL DISPLAY PANEL – 200 mg
NDC 49502-476-26
Rx only
Pretomanid
Tablets
200 mg
Limited
Population*
*See the full prescribing
information for Pretomanid
Tablets 200 mg for information
about the limited population.
Each tablet contains:
Pretomanid 200 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication
out of the reach of children.
Store below 30°C (86°F).
Dispense only in original container.
Keep container tightly closed.
Manufactured by: Mylan Laboratories Limited
Hyderabad - 500 096, India
Manufactured for: Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Under license from TB Alliance.
Code No.: MH/DRUGS/AD/089
MS:MXI:47626:1C:R2
Mylan.com