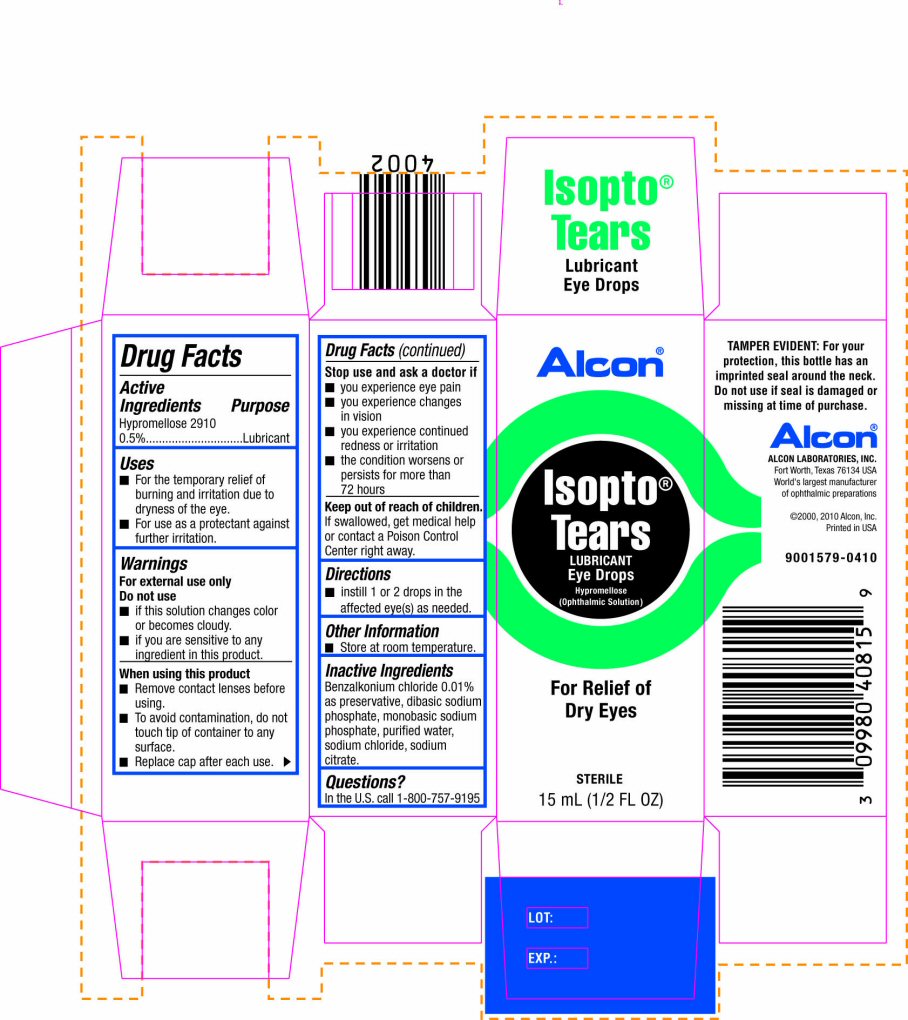
INDICATIONS & USAGE SECTION
Uses
- For the temporary relief of burning and irritation due to dryness of the eye.
- For use as a protectant against further irritation.
WARNINGS SECTION
For external use only
Do not use
- if this solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
OTC - WHEN USING SECTION
When using this product
- Remove contact lenses before using.
- To avoid contamination, do not touch tip of container to any surface.
- Replace cap after each use.
OTC - STOP USE SECTION
Stop use and ask a doctor if
- you experience eye pain
- you experience changes in vision
- you experience continued redness or irritation
- the condition worsens or persists for more than 72 hours
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENT SECTION
Benzalkonium chloride 0.01% as preservative, dibasic sodium phosphate, monobasic sodium phosphate, purified water, sodium chloride, sodium citrate.
PRINCIPAL DISPLAY PANEL
Alcon®
Isopto® Tears
LUBRICANT
Eye Drops
Hypromellose (Ophthalmic Solution)
For Relief of Dry Eyes
STERILE
15 mL (1/2 FL OZ)
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
Alcon®
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
World's largest manufacturer of ophthalmic preparations
©2000, 2010 Alcon, Inc.
Printed in USA
9001579-0410