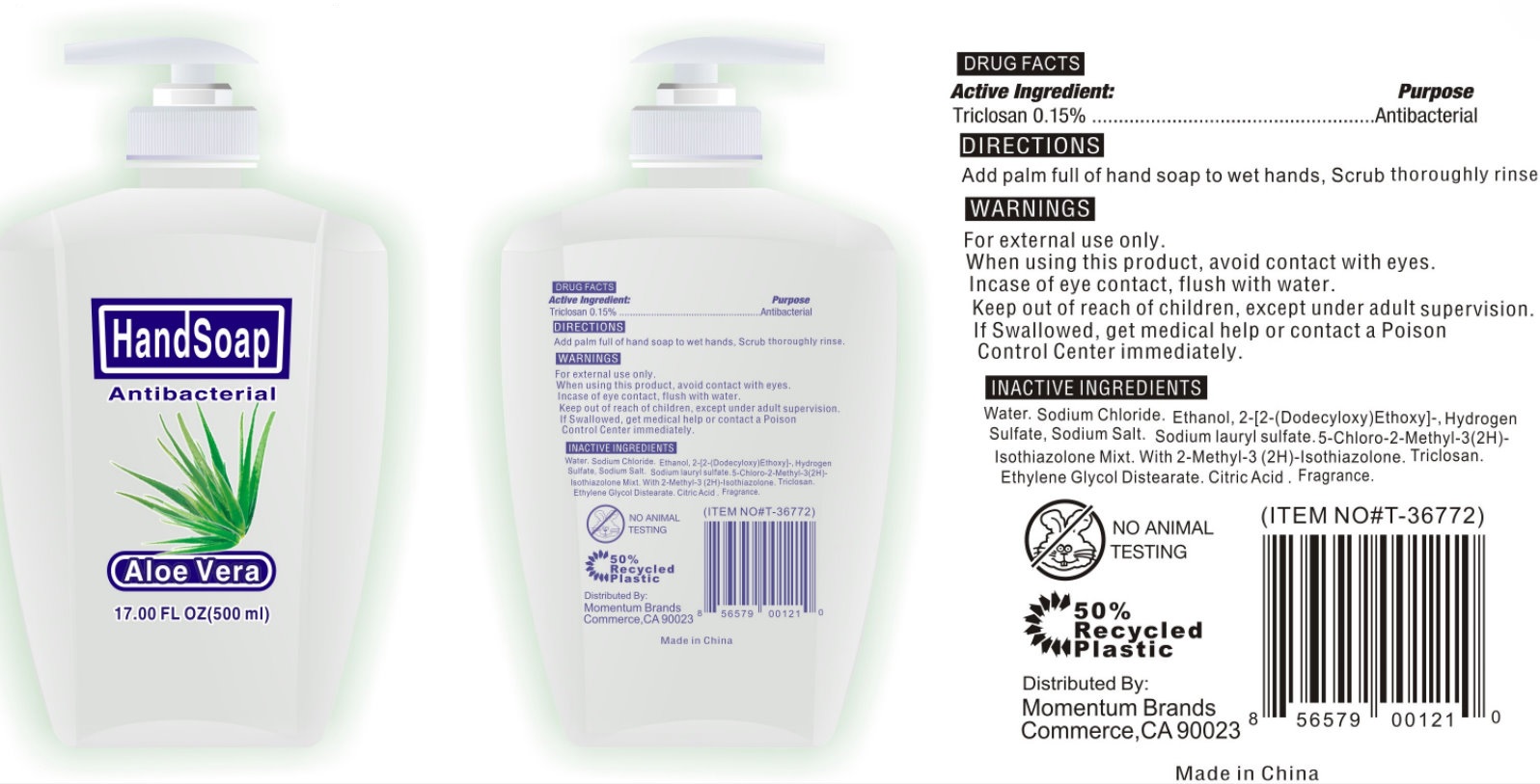
ANTIBACTERIAL HAND - ALOE VERA - triclosan soap
Lonkey Overseas Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient:
Triclosan 0.15%
Uses:
For handwashing to decrease bacteria on the skin
Warnings
For external use only.
When using this product, avoid contact with eyes.
In case of eye contact, flush with water.
Keep out of reach of children, except under adult supervision.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Add palm full of hand soap to wet hands, Scrub thoroughly rinse.
Inactive Ingredients
Distilled Water, Sodium CHloride, Sodium Lauryl Ether Sulfate, N,N-dimethyldodecylamine-N-oxide sol, Lauril Sulfato de Sodio, Sodio C14-16 Olefina Sulfonato, Triclosan, Citric Acid, Mezcla de 5-cloro-2-metil-3(2H)-isotiazolon.2-Metil-2Hisotiazol-3-on, Diestearato de Glicol, Fragrance.
All Rights Reserved
Distributed By:
LONKEY OVERSEAS INCORPORATION
MADE IN CHINA
Antibacterial Hand Soap - Aloe Vera 17oz/500ml (42302-010-00)
Antibacterial
Hand Soap
Aloe Vera
17.00FL OZ (500ml)
Lonkey Overseas Inc.
