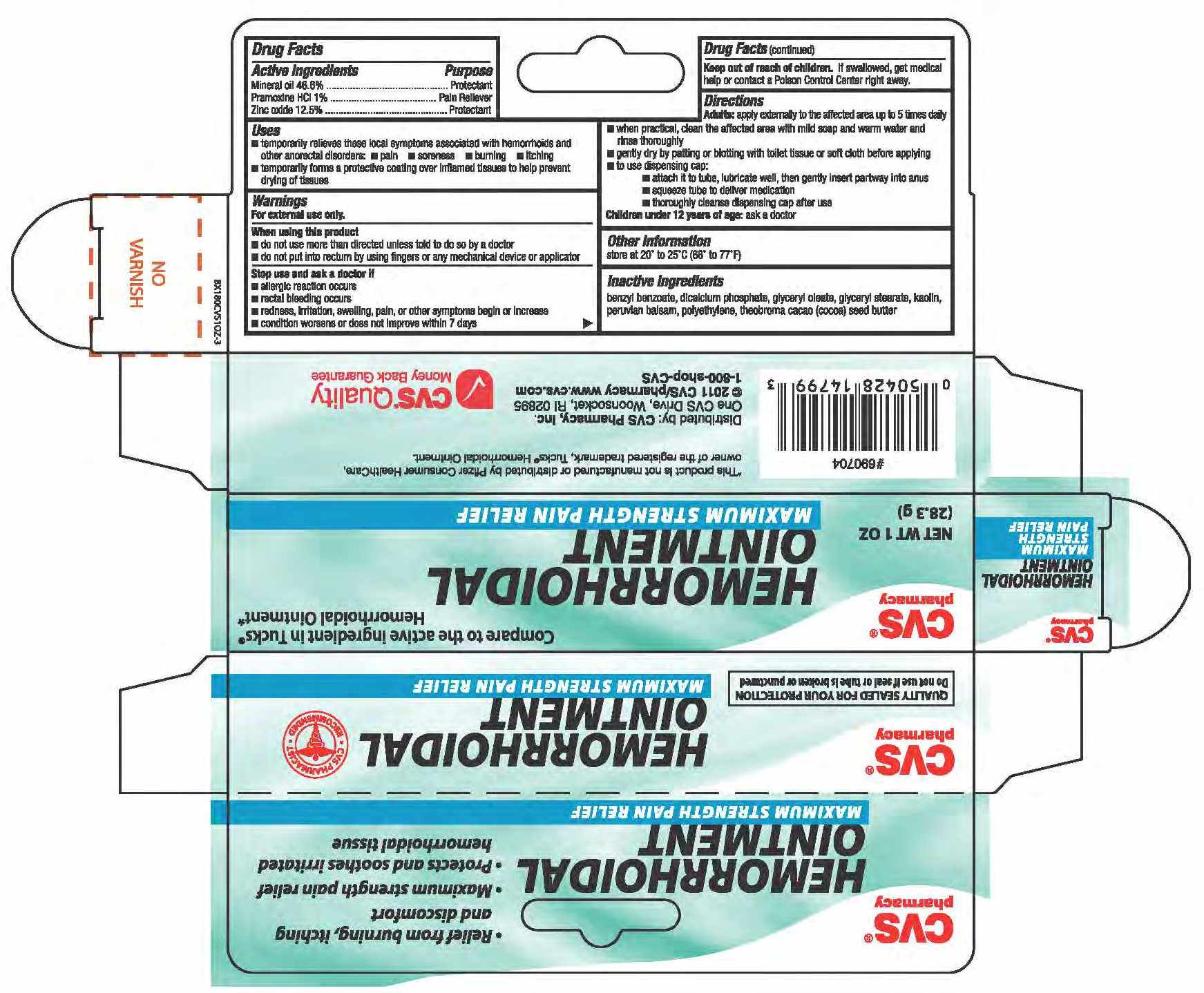
Active ingredients Purpose
Mineral Oil 46.8%.............................................Protectant
Pramoxine HCL 1%......................................... Pain Reliever
Zinc Oxide 12.5%............................................Protectant
Mineral Oil 46.8%.............................................Protectant
Pramoxine HCL 1%......................................... Pain Reliever
Zinc Oxide 12.5%............................................Protectant
Active ingredients Purpose
Mineral Oil 46.8%.............................................Protectant
Pramoxine HCL 1%......................................... Pain Reliever
Zinc Oxide 12.5%............................................Protectant
Uses
- temporarily relieves these local symptoms associated with hemorrhoids and other anorectal disorders:
- pain - soreness - burning - itching
- temporarily forms a protective coating over inflamed tissues to help prevent drying of tissues
Mineral Oil 46.8%.............................................Protectant
Pramoxine HCL 1%......................................... Pain Reliever
Zinc Oxide 12.5%............................................Protectant
Uses
- temporarily relieves these local symptoms associated with hemorrhoids and other anorectal disorders:
- pain - soreness - burning - itching
- temporarily forms a protective coating over inflamed tissues to help prevent drying of tissues
Keep out of reach of children. If swallowed, get medical help or contact
a Poison Control Center right away.
Uses
- temporarily relieves these local symptoms associated with hemorrhoids and other anorectal disorders:
- pain - soreness - burning - itching
- temporarily forms a protective coating over inflamed tissues to help prevent drying of tissues
Other information - store at 20 degrees - 25 degrees C (68 degrees - 77 degrees F)
- temporarily relieves these local symptoms associated with hemorrhoids and other anorectal disorders:
- pain - soreness - burning - itching
- temporarily forms a protective coating over inflamed tissues to help prevent drying of tissues
Other information - store at 20 degrees - 25 degrees C (68 degrees - 77 degrees F)
Warnings
For external use only.
When using this product
- do not use more than directed unless told to do so by a doctor
- do not put into rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- allergic reaction occurs
- rectal bleeding occurs
- redness, irritation, swelling pain or other symptoms begin or increase
- condition worsens, or does not improve within 7 days
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away
For external use only.
When using this product
- do not use more than directed unless told to do so by a doctor
- do not put into rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- allergic reaction occurs
- rectal bleeding occurs
- redness, irritation, swelling pain or other symptoms begin or increase
- condition worsens, or does not improve within 7 days
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
Adults: apply externally to the affected area up to 5 times daily
- when practical, clean the affected area with mild soap and warn water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or soft cloth before applying
- to use dispensing cap:
- attach it to tube, lubricate well, then gently insert partway into anus
- squeeze tube to deliver medication
- thoroughly cleanse dispensing cap after use
Children under 12 years of age: ask a doctor