FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Balsalazide disodium capsules are indicated for the treatment of mildly to moderately active ulcerative colitis in patients 5 years of age and older.
Limitations of Use
Safety and effectiveness of balsalazide beyond 8 weeks in pediatric patients 5 years to 17 years of age and 12 weeks in adults have not been established.
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Evaluate renal function before initiating therapy with balsalazide disodium capsules [see Warnings and Precaution (5.1)].
- Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.
- For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.
- Place a small amount (approximately 10 mL) of applesauce into a clean container.
- Carefully open the capsules.
- Sprinkle the capsule contents on the applesauce.
- Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.
- Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.
- Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.
2.2 Recommended Dosage in Adults and Pediatric Patients 5 Years to 17 Years of Age
Adults
The recommended dosage in adults is 2.25 g (three 750 mg capsules) three times daily for up to 8 weeks. Some patients in the adult clinical trials required treatment for up to 12 weeks.
Pediatric Patients 5 Years to 17 Years of Age
The recommended dosage in pediatric patients 5 years to 17 years of age is either:
-
2.25 g (three 750 mg capsules) three times daily for up to 8 weeks;
OR:
-
750 mg (one capsule) three times daily for up to 8 weeks.
Use of balsalazide in the pediatric population for more than 8 weeks has not been evaluated in clinical trials [see Clinical Studies (14)].
3 DOSAGE FORMS AND STRENGTHS
Balsalazide disodium capsules, USP are available as opaque white capsules containing 750 mg balsalazide disodium, imprinted with “APO B750” in red ink.
4 CONTRAINDICATIONS
Balsalazide disodium capsules is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the components of balsalazide disodium capsules or balsalazide metabolites [see Warnings and Precautions (5.3), Adverse Reactions (6.2), Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure, has been reported in patients given products such as balsalazide disodium capsules that release mesalamine into the gastrointestinal tract. Evaluate renal function prior to initiation of balsalazide disodium capsules and periodically while on therapy. Evaluate the risks and benefits of using balsalazide disodium capsules in patients with known renal impairment, a history of renal disease or taking nephrotoxic drugs. Discontinue balsalazide disodium capsules if renal function deteriorates while on therapy [see Drug Interactions (7.1), Use in Specific Populations (8.6)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Balsalazide is converted to mesalamine, which has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with balsalazide disodium capsules.
5.3 Hypersensitivity Reactions
Some patients have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to balsalazide disodium capsules or to other compounds that contain or are converted to mesalamine.
Mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue balsalazide disodium capsules if an alternative etiology for the signs and symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Because balsalazide is converted to mesalamine, evaluate the risks and benefits of using balsalazide disodium capsules in patients with known liver impairment.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine, the active moiety of balsalazide disodium capsules [see Adverse Reactions (6.2)]. Discontinue balsalazide disodium capsules at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Upper Gastrointestinal Tract Obstruction
Pyloric stenosis or other organic or functional obstruction in the upper gastrointestinal tract may cause prolonged gastric retention of balsalazide disodium capsules, which would delay mesalamine release in the colon. Avoid balsalazide disodium capsules in patients at risk of upper gastrointestinal tract obstruction.
5.7 Photosensitivity
Patients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
5.8 Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, the active moiety of balsalazide disodium capsules, including stones with 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment with balsalazide disodium capsules.
5.9 Interference with Laboratory Tests
Use of balsalazide disodium capsules, which is converted to mesalamine, may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and the main metabolite of mesalamine, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Renal Impairment [see Warnings and Precautions (5.1)]
- Mesalamine-Induced Acute Intolerance Syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Failure [see Warnings and Precautions (5.4)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5)]
- Upper Gastrointestinal Tract Obstruction [see Warnings and Precautions (5.6)]
- Photosensitivity [see Warnings and Precautions (5.7)]
- Nephrolithiasis [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Ulcerative Colitis
During clinical development, 259 adult patients with active ulcerative colitis were exposed to 6.75 g/day balsalazide in 4 controlled trials.
In the 4 controlled clinical trials patients receiving a balsalazide dose of 6.75 g/day most frequently reported the following adverse reactions: headache (8%), abdominal pain (6%), diarrhea (5%), nausea (5%), vomiting (4%), respiratory infection (4%), and arthralgia (4%). Withdrawal from therapy due to adverse reactions was comparable among patients on balsalazide and placebo.
Adverse reactions reported by 1% or more of patients who participated in the 4 well-controlled, Phase 3 trials are presented by treatment group (Table 1).
The number of placebo patients (35), however, is too small for valid comparisons. Some adverse reactions, such as abdominal pain, fatigue, and nausea were reported more frequently in women than in men. Abdominal pain, rectal bleeding, and anemia can be part of the clinical presentation of ulcerative colitis.
Table 1: Adverse Reactions Occurring in ≥ 1 % of Adult Balsalazide Patients in Controlled Trials*
| Adverse Reaction | Balsalazide Capsules 6.75 g/day [N=259] | Placebo [N=35] |
| Abdominal pain Diarrhea Arthralgia Rhinitis Insomnia Fatigue Flatulence Fever Dyspepsia Pharyngitis Coughing Anorexia Urinary tract infection Myalgia Flu-like disorder Dry mouth Cramps Constipation | 16 (6%) 14 (5%) 9 (4%) 6 (2%) 6 (2%) 6 (2%) 5 (2%) 5 (2%) 5 (2%) 4 (2%) 4 (2%) 4 (2%) 3 (1%) 3 (1%) 3 (1%) 3 (1%) 3 (1%) 3 (1%) | 1 (3%) 1 (3%) 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% |
*Adverse reactions occurring in at least 1 % of balsalazide patients which were less frequent than placebo for the same adverse reaction were not included in the table.
Pediatric Ulcerative Colitis
In a clinical trial in 68 pediatric patients aged 5 to 17 years with mildly to moderately active ulcerative colitis who received 6.75 g/day or 2.25 g/day balsalazide disodium for 8 weeks, the most frequently reported adverse reactions were headache (15%), abdominal pain upper (13%), abdominal pain (12%), vomiting (10%), diarrhea (9%), colitis ulcerative (6%), nasopharyngitis (6%), and pyrexia (6%) [see Table 2].
One patient who received balsalazide disodium 6.75 g/day and 3 patients who received balsalazide disodium 2.25 g/day discontinued treatment because of adverse reactions. In addition, 2 patients in each dose group discontinued because of a lack of efficacy.
Adverse reactions reported by 3% or more of pediatric patients within either treatment group in the Phase 3 trial are presented in Table 2.
Table 2: Treatment-Emergent Adverse Reactions Reported by ≥3% of Patients in Either Treatment Group in a Controlled Study of 68 Pediatric Patients
| balsalazide disodium | |||
| Adverse Reaction | 6.75 g/day [N=33] | 2.25 g/day [N=35] | Total [N=68] |
| Headache | 5 (15%) | 5 (14%) | 10 (15%) |
| Abdominal pain upper | 3 (9%) | 6 (17%) | 9 (13%) |
| Abdominal pain | 4 (12%) | 4 (11%) | 8 (12%) |
| Vomiting | 1 (3%) | 6 (17%) | 7 (10%) |
| Diarrhea | 2 (6%) | 4 (11%) | 6 (9%) |
| Colitis ulcerative | 2 (6%) | 2 (6%) | 4 (6%) |
| Nasopharyngitis | 3 (9%) | 1 (3%) | 4 (6%) |
| Pyrexia | 0 (0%) | 4 (11%) | 4 (6%) |
| Hematochezia | 0 (0%) | 3 (9%) | 3 (4%) |
| Nausea | 0 (0%) | 3 (9%) | 3 (4%) |
| Influenza | 1 (3%) | 2 (6%) | 3 (4%) |
| Fatigue | 2 (6%) | 1 (3%) | 3 (4%) |
| Stomatitis | 0 (0%) | 2 (6%) | 2 (3%) |
| Cough | 0 (0%) | 2 (6%) | 2 (3%) |
| Pharyngolaryngeal pain | 2 (6%) | 0 (0%) | 2 (3%) |
| Dysmenorrhea | 2 (6%) | 0 (0%) | 2 (3%) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of balsalazide, or other products which contain or are metabolized to mesalamine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular and Vascular: Myocarditis, pericarditis, vasculitis [see Warnings and Precautions (5.3)]
Respiratory: pleural effusion, pneumonia (with and without eosinophilia), alveolitis, pleurisy/pleuritis
Renal: renal failure, interstitial nephritis, nephrolithiasis [see Warnings and Precautions (5.1, 5.8)]
- Urine discoloration occurring ex-vivo caused by contact of mesalamine, including inactive metabolite, with surfaces or water treated with hypochlorite-containing bleach
Gastrointestinal: pancreatitis
Dermatologic: pruritus, alopecia
Hepatic: hepatotoxicity, elevated liver function tests (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, hepatocellular damage including liver necrosis and liver failure, Kawasaki-like syndrome including hepatic dysfunction
Skin: SJS/TEN, DRESS, and AGEP [see Warnings and Precautions (5.5)]
7 DRUG INTERACTIONS
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs), may increase the risk of renal reactions. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1)].
7.2 Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or any other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of balsalazide disodium capsules and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
7.3 Interference With Urinary Normetanephrine Measurements
Use of balsalazide disodium capsules, which is converted to mesalamine, may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection [see Warnings and Precautions (5.9)]. Consider an alternative, selective assay for normetanephrine.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from meta-analyses, cohort studies and case series on the use of mesalamine, the active moiety of balsalazide, during pregnancy have not reliably informed an association with mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are adverse effects on maternal and fetal outcomes associated with ulcerative colitis in pregnancy (see Clinical Considerations). In animal reproduction studies, there were no adverse developmental effects observed after oral administration of balsalazide disodium in pregnant rats and rabbits during organogenesis at doses up to 2.4 and 4.7 times, respectively, the maximum recommended human dose (MRHD) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2,500 g) infants, and small for gestational age at birth.
Data
Human Data
Published data from meta-analyses, cohort studies and case series on the use of mesalamine, the active moiety of balsalazide, during early pregnancy (first trimester) and throughout pregnancy have not reliably informed an association of mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is no clear evidence that mesalamine exposure in early pregnancy is associated with an increased risk in major congenital malformations, including cardiac malformations. Published epidemiologic studies have important methodological limitations which hinder interpretation of the data, including inability to control for confounders, such as underlying maternal disease, and maternal use of concomitant medications, and missing information on the dose and duration of use for mesalamine products.
Animal Data
Reproduction studies were performed in rats and rabbits following administration of balsalazide during organogenesis at oral doses up to 2 g/kg/day, 2.4 and 4.7 times the MRHD based on body surface area for the rat and rabbit, respectively, and revealed no adverse embryofetal developmental effects due to balsalazide disodium.
8.2 Lactation
Risk Summary
Data from published literature report the presence of mesalamine and its metabolite, N acetyl-5 aminosalicylic acid, in human milk in small amounts with relative infant doses (RID) of 0.1% or less for mesalamine (see Data). There are case reports of diarrhea in breastfed infants exposed to mesalamine (see Clinical Considerations). There is no information on the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of balsalazide to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for balsalazide and any potential adverse effects on the breastfed child from balsalazide or from the underlying maternal condition.
Clinical Considerations
Advise the caregiver to monitor breastfed infants for diarrhea.
Data
In published lactation studies, maternal mesalamine doses from various oral and rectal mesalamine formulations and products ranged from 500 mg to 4.8 g daily. The average concentration of mesalamine in milk ranged from non-detectable to 0.5 mg/L. The average concentration of N-acetyl-5-aminosalicylic acid in milk ranged from 0.2 to 9.3 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.075 mg/kg/day (RID 0 to 0.1%) of mesalamine and 0.03 to 1.4 mg/kg/day of N-acetyl-5-aminosalicylic acid.
8.4 Pediatric Use
The safety and effectiveness of balsalazide disodium has been established for the treatment of mildly to moderately active ulcerative colitis in pediatric and adolescent patients 5 years to 17 years of age. Use of balsalazide disodium for this indication is supported by evidence from adequate and well-controlled clinical studies in adults with additional pharmacokinetic and safety data in pediatric patients aged 5 years to 17 years [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.2)].
Based on the limited data available, dosing can be initiated at either 6.75 or 2.25 g/day [see Dosage and Administration (2.2)].
The safety and effectiveness of balsalazide disodium capsules in pediatric patients below the age of 5 years have not been established.
8.5 Geriatric Use
Clinical trials of balsalazide disodium did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently than younger subjects. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia and pancytopenia, in patients who were 65 years or older compared to younger patients taking mesalamine-containing products. Balsalazide disodium is converted into mesalamine in the colon. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with balsalazide disodium. In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients when prescribing balsalazide disodium capsules [see Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions to balsalazide disodium, which is converted to mesalamine, may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on balsalazide disodium capsules therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue balsalazide disodium capsules if renal function deteriorates while on therapy [see Warnings and Precautions (5.1), Adverse Reactions (6.2), Drug Interactions (7.1)].
10 OVERDOSAGE
Balsalazide disodium capsules is an aminosalicylate, and symptoms of salicylate toxicity include: nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe salicylate intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) damage. There is no specific antidote for balsalazide overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent further absorption. Proper medical care should be sought immediately with appropriate supportive care, including the possible use of emesis, cathartics, and activated charcoal to prevent further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
11 DESCRIPTION
Each balsalazide disodium capsule, USP contains 750 mg of balsalazide disodium, a prodrug that is enzymatically cleaved in the colon to produce mesalamine (5-aminosalicylic acid or 5-ASA), an aminosalicylate. Each capsule of balsalazide (750 mg) is equivalent to 267 mg of mesalamine. Balsalazide disodium has the chemical name (E)-5-[[-4-[[(2- carboxyethyl)amino]carbonyl] phenyl]azo]-2-hydroxybenzoic acid, disodium salt, dihydrate. Its structural formula is:
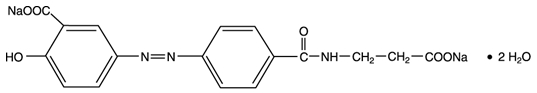
Molecular Weight: 437.31 g/mol
Molecular Formula: C17H13N3O6Na2•2H2O
Balsalazide disodium is a stable, odorless orange to yellow microcrystalline powder. It is freely soluble in water and isotonic saline, sparingly soluble in methanol and ethanol, and practically insoluble in all other organic solvents.
Inactive Ingredients: Each hard gelatin capsule contains colloidal silicon dioxide, gelatin, magnesium stearate and titanium dioxide. The capsule imprinting ink contains propylene glycol, strong ammonia solution, shellac and red iron oxide. The sodium content of each capsule is approximately 86 mg.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Balsalazide disodium is delivered intact to the colon where it is cleaved by bacterial azoreduction to release equimolar quantities of mesalamine, which is the therapeutically active portion of the molecule, and the 4-aminobenzoyl-β-alanine carrier moiety. The carrier moiety released when balsalazide disodium is cleaved is only minimally absorbed and is largely inert.
The mechanism of action of 5-ASA is not fully understood, but appears to be a local anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with ulcerative colitis, and it is possible that 5-ASA diminishes inflammation by blocking production of arachidonic acid metabolites in the colon.
12.3 Pharmacokinetics
Balsalazide disodium capsules contain a powder of balsalazide disodium that is insoluble in acid and designed to be delivered to the colon as the intact prodrug. Upon reaching the colon, bacterial azoreductases cleave the compound to release 5-ASA, the therapeutically active portion of the molecule, and 4-aminobenzoyl-β-alanine. The 5-ASA is further metabolized to yield N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), a second key metabolite.
Absorption
In a study of adult patients with ulcerative colitis, who received balsalazide, 1.5 g twice daily, for over 1 year, systemic drug exposure, based on mean AUC values, was up to 60 times greater (0.008 mcg·hr/mL to 0.480 mcg·hr/mL) when compared to that obtained in healthy subjects who received the same dose.
Effect of Food
The plasma pharmacokinetics of balsalazide and its key metabolites from a crossover study in healthy subjects are summarized in Table 3. In this study, a single oral dose of balsalazide 2.25 g was administered to healthy volunteers as intact capsules (3 x 750 mg) under fasting conditions, as intact capsules (3 x 750 mg) after a high-fat meal, and unencapsulated (3 x 750 mg) as sprinkles on applesauce.
|
Fasting N = 17 |
High-Fat Meal N = 17 |
Sprinkled N = 17 |
|
| Cmax (mcg/mL) | |||
| Balsalazide | 0.51 ± 0.32 | 0.45 ± 0.39 | 0.21 ± 0.12 |
| 5-ASA | 0.22 ± 0.12 | 0.11 ± 0.136 | 0.29 ± 0.17 |
| N-Ac-5-ASA | 0.88 ± 0.39 | 0.64 ± 0.534 | 1.04 ± 0.57 |
| AUClast mcg·hr/mL) | |||
| Balsalazide | 1.35 ± 0.73 | 1.52 ± 1.01 | 0.87 ± 0.48 |
| 5-ASA | 2.59 ± 1.46 | 2.10 ± 2.58 | 2.99 ± 1.70 |
| N-Ac-5-ASA | 17.8 ± 8.14 | 17.7 ± 13.7 | 20 ± 11.4 |
| Tmax (h) | |||
| Balsalazide | 0.8 ± 0.85 | 1.2 ± 1.11 | 1.6 ± 0.44 |
| 5-ASA | 8.2 ± 1.98 | 22 ± 8.23 | 8.7 ± 1.99 |
| N-Ac-5-ASA | 9.9 ± 2.49 | 20.2 ± 8.94 | 10.8 ± 5.39 |
A relatively low systemic exposure was observed under all three administered conditions (fasting, fed with high-fat meal, sprinkled on applesauce), which reflects the variable, but minimal absorption of balsalazide disodium and its metabolites. The data indicate that both Cmax and AUClast were lower, while Tmax was markedly prolonged, under fed (high-fat meal) compared to fasted conditions. Moreover, the data suggest that dosing balsalazide disodium as a sprinkle or as a capsule provides highly variable, but relatively similar mean pharmacokinetic parameter values. No inference can be made as to how the systemic exposure differences of balsalazide and its metabolites in this study might predict the clinical efficacy under different dosing conditions (i.e., fasted, fed with high-fat meal, or sprinkled on applesauce) since clinical efficacy after balsalazide disodium administration is presumed to be primarily due to the local effects of 5-ASA on the colonic mucosa [see Dosage and Administration (2.1)].
Distribution
The binding of balsalazide to human plasma proteins was ≥ 99%.
Elimination
Metabolism
The products of the azoreduction of this compound, 5-ASA and 4-aminobenzoyl-β-alanine, and their N-acetylated metabolites have been identified in plasma, urine and feces.
Excretion
Following single-dose administration of 2.25 g balsalazide (three 750 mg capsules) under fasting conditions in healthy subjects, mean urinary recovery of balsalazide, 5-ASA, and N-Ac-5-ASA was 0.20%, 0.22% and 10.2%, respectively.
In a multiple-dose study in healthy subjects receiving a balsalazide dose of two 750 mg capsules twice daily (3 g/day) for 10 days, mean urinary recovery of balsalazide, 5-ASA, and N-Ac-5-ASA was 0.1%, 0%, and 11.3%, respectively. During this study, subjects received their morning dose 0.5 hours after being fed a standard meal, and subjects received their evening dose 2 hours after being fed a standard meal.
In a study with 10 healthy subjects, 65% of a single 2.25-gram dose of balsalazide was recovered as 5-ASA, 4-aminobenzoyl-β-alanine, and the N-acetylated metabolites in feces, while <1% of the dose was recovered as parent compound.
In a study that examined the disposition of balsalazide in patients who were taking 3 to 6 g of balsalazide daily for more than 1 year and who were in remission from ulcerative colitis, less than 1% of an oral dose was recovered as intact balsalazide in the urine. Less than 4% of the dose was recovered as 5-ASA, while virtually no 4-aminobenzoyl-β-alanine was detected in urine. The mean urinary recovery of N-Ac-5-ASA and N-acetyl-4-aminobenzoyl-β-alanine comprised <16% and <12% of the balsalazide dose, respectively. No fecal recovery studies were performed in this population.
Use in Specific Populations
Pediatric Patients
In studies of pediatric patients with mild-to-moderate active ulcerative colitis receiving three 750 mg balsalazide disodium capsules 3 times daily (6.75 g/day) for 8 weeks, steady state was reached within 2 weeks, as observed in adult patients. Likewise, the pharmacokinetics of balsalazide, 5-ASA, and N-Ac-5-ASA were characterized by very large inter-patient variability, which is also similar to that seen in adult patients.
The prodrug moiety, balsalazide, appeared to exhibit dose-independent (i.e., dose-linear) kinetics in children, and the systemic exposure parameters (Cmax and AUC0 to 8) increased in an almost dose-proportional fashion after the 6.75 g/day versus the 2.25 g/day doses. However, the absolute magnitude of these exposure parameters was greater relative to adults. The Cmax and AUC0 to 8 observed in pediatric patients were 26% and 102% greater than those observed in adult patients at the 6.75 g/day dosage level. In contrast, the systemic exposure parameters for the active metabolites, 5-ASA and N-Ac- 5-ASA, in pediatric patients increased in a less than dose-proportional manner after the 6.75 g/day dose versus the 2.25 g/day dose. Additionally, the magnitude of these exposure parameters was decreased for both metabolites relative to adults. For the metabolite of key safety concern from a systemic exposure perspective, 5-ASA, the Cmax and AUC0 to 8 observed in pediatric patients were 67% and 64% lower than those observed in adult patients at the 6.75 g/day dosage level. Likewise, for N-Ac-5-ASA, the Cmax and AUC0 to 8 observed in pediatric patients were 68% and 55% lower than those observed in adult patients at the 6.75 g/day dosage level.
All pharmacokinetic studies with balsalazide are characterized by large variability in the plasma concentration versus time profiles for balsalazide and its metabolites, thus half-life estimates of these analytes are indeterminate.
Drug Interaction Studies
In Vitro Data
In an in vitro study using human liver microsomes, balsalazide and its metabolites [5-aminosalicylic acid (5-ASA), N acetyl-5-aminosalicylic acid (N-Ac-5-ASA), 4-aminobenzoyl-ß-alanine (4-ABA) and N-acetyl-4-aminobenzoyl-ß-alanine (N-Ac-4-ABA)] were not shown to inhibit the major CYP enzymes evaluated (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5). Therefore, balsalazide and its metabolites are not expected to inhibit the metabolism of other drugs which are substrates of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month rat (Sprague Dawley) carcinogenicity study, oral (dietary) balsalazide disodium at doses up to 2 g/kg/day was not tumorigenic. For a 50-kg person of average height this dose represents 2.4 times the recommended human dose on a body surface area basis. Balsalazide disodium was not genotoxic in the following in vitro or in vivo tests: Ames test, human lymphocyte chromosomal aberration test, and mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or mouse micronucleus test. However, it was genotoxic in the in vitro Chinese hamster lung cell (CH V79/HGPRT) forward mutation test.
4-aminobenzoyl-β-alanine, a metabolite of balsalazide disodium, was not genotoxic in the Ames test and the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test but was positive in the human lymphocyte chromosomal aberration test. N-acetyl-4-aminobenzoyl-β-alanine, a conjugated metabolite of balsalazide disodium, was not genotoxic in Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or the human lymphocyte chromosomal aberration test. Balsalazide disodium at oral doses up to 2 g/kg/day, 2.4 times the recommended human dose based on body surface area, was found to have no effect on fertility and reproductive performance in rats.
13.2 Animal Toxicology and/or Pharmacology
Renal Toxicity
In animal studies conducted at doses up to 2,000 mg/kg (approximately 21 times the recommended 6.75 g/day dose on a mg/kg basis for a 70 kg person), balsalazide demonstrated no nephrotoxic effects in rats or dogs.
Overdosage
A single oral dose of balsalazide disodium at 5 g/kg or 4-aminobenzoyl-β-alanine, a metabolite of balsalazide disodium, at 1 g/kg was non-lethal in mice and rats. No symptoms of acute toxicity were seen at these doses.
14 CLINICAL STUDIES
Adult Studies
Two randomized, double-blind studies were conducted in adults. In the first trial, 103 patients with active mild-to-moderate ulcerative colitis with sigmoidoscopy findings of friable or spontaneously bleeding mucosa were randomized and treated with balsalazide 6.75 g/day or balsalazide 2.25 g/day. The primary efficacy endpoint was reduction of rectal bleeding and improvement of at least one of the other assessed symptoms (stool frequency, patient functional assessment, abdominal pain, sigmoidoscopic grade, and physician’s global assessment [PGA]). Outcome assessment for rectal bleeding at each interim period (weeks 2, 4, and 8) encompassed a 4-day period (96 hours). Results demonstrated a statistically significant difference between high and low doses of balsalazide (Figure 1).
Figure 1: Percentage of Patients Improved at 8 Weeks
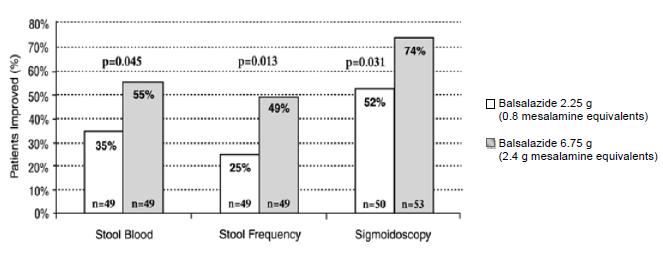
A second study, conducted in Europe, confirmed findings of symptomatic improvement.
Pediatric Studies
A clinical trial was conducted comparing two doses (6.75 g/day and 2.25 g/day) of balsalazide disodium in 68 pediatric patients (age 5 to 17, 23 males and 45 females) with mildly to moderately active ulcerative colitis. 28/33 (85%) patients randomized to 6.75 g/day and 25/35 (71%) patients randomized to 2.25 g/day completed the study. The primary endpoint for this study was the proportion of subjects with clinical improvement (defined as a reduction of at least 3 points in the Modified Sutherland Ulcerative Colitis Activity Index [MUCAI] from baseline to 8 weeks). Fifteen (45%) patients in the balsalazide disodium 6.75 g/day group and 13 (37%) patients in the balsalazide disodium 2.25 g/day group showed this clinical improvement. In both groups, patients with higher MUCAI total scores at baseline were likely to experience greater improvement.
Rectal bleeding improved in 64% of patients treated with balsalazide disodium 6.75 g/day and 54% of patients treated with balsalazide disodium 2.25 g/day. Colonic mucosal appearance upon endoscopy improved in 61% of patients treated with balsalazide disodium 6.75 g/day and 46% of patients treated with balsalazide disodium 2.25 g/day.
16 HOW SUPPLIED/STORAGE AND HANDLING
Balsalazide Disodium Capsules, USP are available as white, opaque capsules imprinted “APO B750” in red ink.
Balsalazide Disodium Capsules, USP are supplied as follows:
Bottles of 30 (NDC 60505-2575-3)
Bottles of 280 (NDC 60505-2575-7)
Bottles of 350 (NDC 60505-2575-4)
Unit Dose Blister Packs of 10 strips of 10 capsules (NDC 60505-2575-0)
Storage
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted between 15ºC and 30ºC (59ºF and 86ºF).
See USP Controlled Room Temperature.
17 PATIENT COUNSELING INFORMATION
Renal Impairment
Inform patients that balsalazide disodium capsules may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, including NSAIDs, and periodic monitoring of renal function will be performed while they are on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Mesalamine-Induced Acute Intolerance Syndrome and Other Hypersensitivity Reactions
Inform patients of the signs and symptoms of hypersensitivity reactions. Instruct patients to stop taking balsalazide disodium capsules and report to their healthcare provider if they experience new or worsening symptoms of Acute Intolerance Syndrome (cramping, abdominal pain, bloody diarrhea, fever, headache, and rash) or other symptoms suggestive of mesalamine-induced hypersensitivity [see Warnings and Precautions (5.2, 5.3)].
Hepatic Failure
Inform patients with known liver disease of the signs and symptoms of worsening liver function and advise them to report to their healthcare provider if they experience such signs or symptoms [see Warnings and Precautions (5.4)].
Severe Cutaneous Adverse Reactions
Inform patients of the signs and symptoms of severe cutaneous adverse reactions. Instruct patients to stop taking balsalazide disodium capsules and report to their healthcare provider at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity [see Warnings and Precautions (5.5)].
Upper Gastrointestinal Tract Obstruction
Advise patients to contact their healthcare provider if they experience signs and symptoms of upper gastrointestinal tract obstruction [see Warnings and Precautions (5.6)].
Photosensitivity
Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors [see Warnings and Precautions (5.7)].
Nephrolithiasis
Instruct patients to drink an adequate amount of fluids during treatment in order to minimize the risk of kidney stone formation and to contact their healthcare provider if they experience signs or symptoms of a kidney stone (e.g., severe side or back pain, blood in the urine) [see Warnings and Precautions (5.8)].
Blood Disorders
Inform elderly patients and those taking azathioprine or 6-mercaptopurine of the risk for blood disorders and the need for periodic monitoring of complete blood cell counts and platelet counts while on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Drug Interactions (7.2), Use in Specific Populations (8.5)].
Administration
Instruct patients:
- Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.
- For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.
- Place a small amount (approximately 10 mL) of applesauce into a clean container.
- Carefully open the capsules.
- Sprinkle the capsule contents on the applesauce.
- Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.
- Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.
- Teeth and/or tongue staining may occur in some patients who use balsalazide disodium capsules in sprinkle form with food.
- Drink an adequate amount of fluids.
- Take balsalazide disodium capsules without regard to meals [see Clinical Pharmacology (12.3)].
- Urine may become discolored reddish-brown while taking balsalazide disodium capsules when it comes in contact with surfaces or water treated with hypochlorite-containing bleach. If discolored urine is observed, advise patients to observe their urine flow. Report to the healthcare provider only if urine is discolored on leaving the body, before contact with any surface or water (e.g., in the toilet).
APOTEX INC.
BALSALAZIDE DISODIUM CAPSULES, USP 750 mg
| Manufactured by | Manufactured for |
| Apotex Inc. | Apotex Corp. |
| Toronto, Ontario | Weston, Florida |
| Canada M9L 1T9 | USA 33326 |
Revision: 13
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 750 mg BOTTLE LABEL
APOTEX CORP. NDC 60505-2575-7
Balsalazide Disodium Capsules
750 mg
Rx
280 count bottle