Uses
temporarily relieves pain and itching due to
- minor cuts
- minor scrapes
- minor burns
- sunburn
- minor skin irritations
- insect bites
Warnings
For external use only.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years and older: Apply externally to the affected area up to 3 to 4 times a day
- Children under 2 years: Consult a doctor
Other Information
- May be applied under occlusive dressing.
-
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Inactive ingredients
benzyl alcohol, carbomer 940, cholesterol, hydrogenated lecithin, polysorbate 80, propylene glycol, trolamine, vitamin E acetate, water
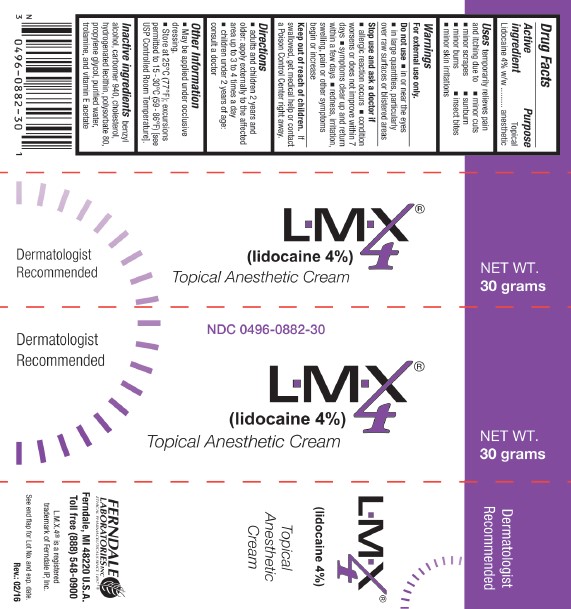
Package Labels-Principal Display Panels
Manufactured by Ferndale Laboratories, Inc.
Ferndale, MI 48220 U.S.A.
Toll free (888) 548-0900
www.ferndalelabs.com
L.M.X.4® is a registered trademark of Ferndale IP, Inc.
Tegaderm™ is a trademark of 3M Corporation.
NDC 0496-0882-06 L.M.X.4® 5 x 5 grams

NDC 0496-0882-07 L.M.X.4® 5 x 5 grams with 10 3M Tegaderm™ Transparent Dressings

NDC 0496-0882-15 L.M.X.4® 15 grams

NDC 0496-0882-30 L.M.X.4® 30 grams
NDC 0496-0882-71 L.M.X.4® 30 grams with 10 3M Tegaderm™ Transparent Dressings

NDC 0496-0882-05 L.M.X.4 5 grams
