FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Jivi, antihemophilic factor (recombinant), PEGylated-aucl, is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and adolescents (12 years of age and older) with hemophilia A (congenital Factor VIII deficiency) for:
- •
- On-demand treatment and control of bleeding episodes
- •
- Perioperative management of bleeding
- •
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitations of Use
Jivi is not indicated for use in children < 12 years of age due to greater risk for hypersensitivity reactions [see Use in Specific Populations (8.4)]. Jivi is not indicated for use in previously untreated patients (PUPs).
Jivi is not indicated for the treatment of von Willebrand disease.
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dose
- •
- Each vial label of Jivi states the Factor VIII potency in international units (IU). One IU is defined by the current WHO (World Health Organization) international standard for Factor VIII concentrate.
- •
- Dosage and duration of treatment depend on the severity of the Factor VIII deficiency, the location and extent of bleeding, and the patient’s clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- •
- Potency assignment for Jivi is determined using a chromogenic substrate assay.
- •
- Monitor the Factor VIII activity of Jivi in plasma using either a validated chromogenic substrate assay or a validated one-stage clotting assay [see Warnings and Precautions (5.4)].
- •
- Calculation of the required dose of Factor VIII is based on the empirical finding that 1 IU of Factor VIII per kilogram body weight increases the plasma Factor VIII level by 2 IU/dL.
- •
- Estimate the required dose for on-demand treatment and control of bleeding and perioperative management using the following formula:
Required dose (IU) = body weight (kg) x desired Factor VIII rise (% of normal or IU/dL)
x reciprocal of expected recovery (or observed recovery, if available) (e.g., 0.5 for a recovery of 2 IU/dL per IU/kg)
- Estimate the expected in vivo peak increase using the following formula:
-
Estimated increment of Factor VIII (IU/dL or % of normal) = [Total dose (IU)/body weight (kg)]
x 2 (IU/dL per IU/kg)
- 1.
- Adjust dose and frequency to the patient’s clinical response. Patients may vary in their pharmacokinetic [e.g., half-life, incremental recovery and AUC (area under the curve)] and clinical responses to Jivi.
- 2.
- The total recommended maximum dose per infusion is approximately 6000 IU (rounded to vial size) [see Clinical Studies (14)].
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing Jivi for the on-demand treatment and control of bleeding episodes is provided in Table 1. The goal of treatment is to maintain a plasma Factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 1.
|
Degree of Bleeding Hemorrhage/Hemorrhagic Event |
Factor VIII Level Required
|
Dose (IU/kg) |
Frequency of Doses (hours) |
Duration of Treatment |
|
Minor (e.g., early hemarthrosis, minor muscle bleeding, oral bleeds) |
20–40 |
10–20 |
Repeat every |
Until bleeding is resolved |
|
Moderate (e.g., more extensive hemarthrosis, muscle bleeding, or hematoma) |
30–60 |
15–30 |
Repeat every |
Until bleeding is resolved |
|
Major (e.g., intracranial, intra-abdominal or intrathoracic hemorrhages, gastrointestinal bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces, or iliopsoas sheath, life- or limb- threatening hemorrhage) |
60–100 |
30–50 |
Repeat every |
Until bleeding is resolved |
Perioperative Management of Bleeding
A guide for dosing Jivi during surgery (perioperative management) is provided in Table 2. The goal of treatment is to maintain a plasma Factor VIII activity level at or above the plasma level (in % of normal or in IU/dL) outlined in Table 2. During major surgery, monitoring with appropriate laboratory tests, including serial Factor VIII activity assays, is highly recommended [see Warnings and Precautions (5.4)].
|
Type of Surgery |
Factor VIII Level Required
|
Dose (IU/kg) |
Frequency of Doses (hours) |
Duration of Treatment (days) |
|
Minor (e.g., tooth extraction) |
30–60 |
15-30 |
Repeat every 24 hours |
At least 1 day until healing is achieved |
|
Major (e.g., intracranial, intra-abdominal, intrathoracic, or joint replacement surgery) |
80–100 |
40-50 |
Repeat every |
Until adequate wound healing is complete, then continue therapy for at least another 7 days to maintain Factor VIII activity of |
2.2 Preparation and Reconstitution
Reconstitute and administer Jivi with the components provided with each package. If any component of the package is opened or damaged, do not use this component.
Reconstitution
Work on a clean surface and wash hands thoroughly using soap and warm water before performing the procedures.
2.3 Administration
For intravenous use only.
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
- •
- Do not use if you notice any particulate matter or discoloration and immediately contact Bayer Medical Communications at 1-888-84-BAYER (1-888-842-2937).
- •
- Administer reconstituted Jivi as soon as possible. If not, store at room temperature for no longer than 3 hours.
- •
- Infuse Jivi intravenously over a period of 1 to 15 minutes. Adapt the rate of administration to the response of each individual patient (maximum infusion rate 2.5 mL/min).
3 DOSAGE FORMS AND STRENGTHS
Jivi is available as a white to slightly yellow lyophilized powder in single-use glass vials containing nominally 500, 1000, 2000, or 3000 IU of Factor VIII potency per vial.
Each vial of Jivi is labeled with actual Factor VIII potency expressed in IU determined using a chromogenic substrate assay. This potency assignment employs a Factor VIII concentrate standard that is referenced to the current WHO International Standard for Factor VIII concentrate, and is evaluated by appropriate methodology to ensure accuracy of the results.
4 CONTRAINDICATIONS
Jivi is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product [see Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including severe allergic reactions, have occurred with Jivi. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
Jivi may contain trace amounts of mouse and hamster proteins [see Description (11)]. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG) [see Warnings and Precautions (5.3)].
5.2 Neutralizing Antibodies
Neutralizing antibody (inhibitor) formation can occur following administration of Jivi. Carefully monitor patients for the development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody) [see Warnings and Precautions (5.4)].
5.3 Immune Response to PEG
A clinical immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has been observed primarily in patients < 6 years of age [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)]. The symptoms of the clinical immune response were transient. Anti-PEG IgM titers decreased over time to undetectable levels. No immunoglobulin class switching was observed.
In case of clinical suspicion of loss of drug effect, conduct testing for Factor VIII inhibitors [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)] and Factor VIII recovery.
A low post-infusion Factor VIII level in the absence of detectable Factor VIII inhibitors indicates that loss of drug effect is likely due to anti‑PEG antibodies. Discontinue Jivi and switch patients to a previously effective Factor VIII product.
5.4 Monitoring Laboratory Tests
- •
- If monitoring of Factor VIII activity is performed, use a validated chromogenic assay or a selected validated one-stage clotting assay [see Dosage and Administration (2.1)].
- •
- Laboratories intending to measure the Factor VIII activity of Jivi should check their procedures for accuracy. For Jivi, select silica-based one-stage assays may underestimate the Factor VIII activity of Jivi in plasma samples; some reagents, e.g., with kaolin-based activators, have the potential for overestimation1. Therefore, the suitability of the assay must be ascertained. If a validated one-stage clotting or chromogenic assay is not available locally, then use of a reference laboratory is recommended.
- •
- Monitor for development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected Factor VIII plasma levels are not attained or if bleeding is not controlled with the expected dose of Jivi. Use Bethesda Units (BU) to report inhibitor titers.
6 ADVERSE REACTIONS
The most frequently (≥ 5%) reported adverse reactions in clinical trials in previously treated patients (PTPs) ≥ 12 years of age were headache, cough, nausea and fever (see Table 3) .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 221 subjects constituted the safety population from three studies. Subjects who received Jivi for perioperative management (n=17) with treatment period of 2 to 3 weeks were excluded from pooled safety analysis but included in analysis for inhibitor development. The median EDs for adults and adolescents (≥ 12 years of age) was 131 EDs (range: 1–309) per subject; and the median EDs for subjects < 12 years of age was 53 EDs (range: 1–68) per subject.
|
MedDRA Standard System Organ Class Preferred term |
All Subjects n (%) n=221 |
Subjects ≥12 years of age n (%) n=148 |
|
Gastrointestinal Disorders | ||
|
Abdominal pain |
9 (4%) |
5 (3%) |
|
Nausea |
9 (4%) |
8 (5%) |
|
Vomiting |
10 (5%) |
5 (3%) |
|
General Disorders and Administration Site Conditions | ||
|
Injection site reactions1 |
4 (2%) |
2 (1%) |
|
Pyrexia (fever) |
20 (9%) |
8 (5%) |
|
Immune System Disorders | ||
|
Hypersensitivity |
8 (4%) |
3 (2%) |
|
Nervous System Disorders | ||
|
Dizziness |
3 (1%) |
3 (2%) |
|
Dysgeusia (distorted sense of taste) |
1 (1%) |
0 |
|
Headache |
29 (13%) |
21 (14%) |
|
Psychiatric Disorders | ||
|
Insomnia |
5 (2%) |
4 (3%) |
|
Respiratory, Thoracic and Mediastinal Disorders | ||
|
Cough |
18 (8%) |
10 (7%) |
|
Skin and Subcutaneous Tissue Disorders | ||
|
Erythema2 (redness) |
3 (1%) |
2 (1%) |
|
Pruritus (itching) |
2 (1%) |
1 (1%) |
|
Rash3 |
9 (4%) |
3 (2%) |
|
Vascular Disorders | ||
|
Flushing |
1 (1%) |
1 (1%) |
1 Includes Injection site pruritus and Injection site rash
2 Includes Erythema and Erythema multiforme
3 Includes Rash and Rash papular
Immunogenicity
Immunogenicity was evaluated during clinical trials with Jivi in 158 (including surgery subjects) previously treated adult and adolescent (≥ 12 years of age) severe hemophilia A (Factor VIII activity < 1%) subjects with previous exposure to Factor VIII concentrates ≥ 150 EDs. There were 73 previously treated pediatric subjects < 12 years of age [see Use in Specific Populations (8.4)].
Factor VIII Inhibitors
A Factor VIII inhibitor (1.7 BU/mL) was reported in one previously treated adult subject. Repeat testing did not confirm the presence of a Factor VIII inhibitor.
Anti-PEG Antibodies
Immunogenicity against PEG was evaluated by anti-PEG screening and specific IgM anti-PEG ELISA assays. One subject (19 years of age) with asthma, presented at 4 exposure days (EDs) with a clinical hypersensitivity reaction after infusion of Jivi. The subject reported headache, abdominal pain, shortness of breath, and flushing, all of which resolved following his standard asthma treatment. No further medical intervention was required. The event was associated with a transient increase of IgM anti-PEG antibody titer, which was negative upon retest during follow-up within 30 days.
The detection of antibody formation depends on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to Jivi with the incidence of antibodies to other products.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with Jivi use in pregnant women to inform on drug-associated risk. Animal developmental and reproductive toxicity studies have not been conducted with Jivi. It is not known whether Jivi can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of Jivi in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Jivi and any potential adverse effects on the breastfed infant from Jivi or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness in patients below the age of 12 have not been established.
Jivi is not indicated for use in previously untreated patients.
Jivi is not indicated for use in children below 12 years of age [see Clinical Studies (14)].
In completed clinical studies with 73 pediatric previously treated patients (PTPs) < 12 years of age (44 PTPs < 6 years, 29 PTPs 6 to < 12 years), adverse reactions due to immune response to PEG were observed in children less than 6 years of age. In 23% of subjects in the age group < 6 years of age, loss of drug effect due to neutralizing anti-PEG IgM antibodies during the first 4 exposure days (EDs) was observed. In 7% of the subjects < 6 years of age, loss of drug effect was combined with hypersensitivity reactions [see Warnings and Precautions (5.3)].
8.5 Geriatric Use
Clinical studies of Jivi did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease and other drug therapy.
11 DESCRIPTION
Jivi [antihemophilic factor (recombinant), PEGylated-aucl] is a sterile, nonpyrogenic, preservative-free, white to slightly yellow lyophilized powder for reconstitution with sterile Water for Injection (sWFI) as diluent for intravenous (IV) administration. The product is supplied in single-use vials containing dosage strengths of 500, 1000, 2000 and 3000 IU in 2.5 mL fill size. For each dosage strength, the actual assayed potency is directly printed on each vial label. The container closure system consists of a 10 mL, Type I glass vial sealed with a bromobutyl grey stopper and an aluminum crimp seal with plastic flip-off cap plus vial adapter. The vial adapter was designed to connect with the sWFI, prefilled diluent syringe. Jivi is formulated with the following excipients: 59 mg glycine, 27 mg sucrose, 8.4 mg histidine, 4.7 mg sodium chloride, 0.7 mg calcium chloride, and 0.216 mg polysorbate 80. The pH of the reconstituted product is 6.6 to 7.0.
The specific activity of Jivi is approximately 10,000 IU/mg protein.
The active protein (or starting molecule), prior to conjugation is a recombinant B-domain deleted human coagulation Factor VIII (BDD-rFVIII) produced by recombinant DNA technology in Baby Hamster Kidney (BHK) cells.
Jivi is produced by site-specific conjugation of the BDD-rFVIII variant K1804C at the cysteine amino acid position 1804 (within the A3 domain) with a single maleimide-derivatized, 60 kilodalton (kDa) branched PEG (two 30 kDa PEG) moiety. The A3 domain was selected for conjugation to provide both a consistent coagulation activity and high PEGylation efficiency.
The molecular weight of Jivi is approximately 234 kDa based on the calculated average molecular weight of the BDD-rFVIII variant of 165 kDa, plus glycosylation (~4 kDa), and the average molecular weight of the PEG-maleimide of approximately 60 kDa. Functional characterization of Jivi shows comparable mechanism of action to that of rFVIII product with an extended plasma half-life [see Clinical Pharmacology (12.1)].
The manufacturing process of Jivi involves propagation of the recombinant production cell line with the harvest isolation process consisting of continuous filtration of tissue culture fluid and anion exchange chromatography on a membrane adsorber capsule. The process intermediate is purified from process- and product-related impurities using a series of chromatography and filtration steps, including 20 nm viral filtration, prior to conjugation to the 60 kDa maleimide PEG moiety. The mono-PEGylated Jivi active molecule is separated from product-related species by chromatography and then formulated by ultrafiltration. The cell culture, PEGylation, purification process and formulation used in the manufacture of Jivi do not use any additives of human or animal origins.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Jivi, a site-specifically PEGylated recombinant antihemophilic factor [see Description (11)], temporarily replaces the missing coagulation Factor VIII. The site-specific PEGylation in the A3 domain reduces binding to the physiological Factor VIII clearance receptors resulting in an extended half-life and increased AUC [see Clinical Pharmacology (12.3)].
12.2 Pharmacodynamics
The aPTT is prolonged in people with hemophilia A.
Determination of aPTT is a conventional in vitro assay for biological activity of Factor VIII. Treatment with Jivi normalizes the aPTT similar to that achieved with plasma-derived Factor VIII. The administration of Jivi increases plasma levels of Factor VIII and can temporarily correct the coagulation defect in hemophilia A patients.
12.3 Pharmacokinetics
The PK of Jivi was evaluated in two cohorts after single doses of 25 IU/kg and 60 IU/kg and after 25 IU/kg given twice weekly and 60 IU/kg given once weekly for 8 weeks.
The PK profile obtained at Week 8, after repeated dosing, was comparable with the PK profile obtained after the first dose.
In Study 1, the PK of Jivi was investigated in 22 previously treated severe Hemophilia A patients (≥ 12 years of age) following administration of a single dose, 60 IU/kg, of Jivi prior to initiation of prophylactic treatment and in 16 subjects after 6 months of prophylaxis treatment with Jivi. Table 4 summarizes the PK parameters after a single dose, based on plasma Factor VIII activity measured by the chromogenic and one-stage assay.
|
Chromogenic assay |
One-stage assay |
|||
|
PK Parameters (unit) |
25 IU/kg |
60 IU/kg* |
25 IU/kg |
60 IU/kg* |
|
n=7 |
n=29 |
n=7 |
n=29 |
|
|
AUC (IU*h/dL) |
1640 ± 550 |
4060 ± 1420 |
1640 ± 660 |
4150 ± 1060 |
|
Cmax (IU/dL) |
64.2 ± 9.2 |
167 ± 30 |
69.4 ± 11.3 |
213 ± 71 |
|
t½ (h) |
18.6 ± 4.6 |
17.9 ± 4.0 |
21.4 ± 13.1 |
17.4 ± 3.8 |
|
MRTIV (h) |
26.7 ± 6.6 |
25.8 ± 5.9 |
29.0 ± 14.0 |
24.5 ± 5.4 |
|
Vss (mL/kg) |
42.8 ± 5.0 |
39.4 ± 6.3 |
44.7 ± 5.4 |
36.0 ± 6.5 |
|
CL (mL/h) |
142 ± 33 |
121 ± 53 |
146 ± 44 |
114 ± 41 |
|
CL (mL/h/kg) |
1.68 ± 0.39 |
1.63 ± 0.52 |
1.74 ± 0.54 |
1.52 ± 0.38 |
|
Recovery [(IU/dL)/(IU/kg)] |
2.13 ± 0.47 |
2.53 ± 0.43† |
2.21 ± 0.55 |
3.25 ± 0.84† |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals to evaluate the carcinogenic or genotoxic potential of Jivi, or studies to determine the effects of Jivi on fertility, have not been performed. No effect on male and female reproductive organs was seen in repeated-administration toxicity studies. Genotoxicity studies conducted with the PEG component of Jivi showed no indication of genotoxicity.
13.2 Animal Toxicology and/or Pharmacology
No adverse effects were observed in immune-deficient rats intravenously injected with Jivi (40-1200 IU/kg/injection), twice weekly for 26 weeks. No evidence of accumulation of the PEG component of Jivi was detected by immunohistochemical staining in the brain (including the choroid plexus), spleen, or kidneys in animals sacrificed at 13 and 26 weeks.
14 CLINICAL STUDIES
The efficacy of Jivi for on-demand treatment, perioperative management of bleeding and routine prophylaxis in male subjects with severe hemophilia A were evaluated in one international (including U.S.) clinical study in subjects ≥ 12 years. Immunocompetent subjects with severe hemophilia A (Factor VIII activity <1%) and no history of Factor VIII inhibitors were eligible for the trial.
Study 1 (NCT01580293): A multinational, open-label, uncontrolled, partially randomized study in adolescent and adult (12 to 65 years of age) previously treated patients (PTPs) (≥ 150 exposure days [EDs]) consisted of three parts: Part A (Weeks 0 – 36)3; an optional extension phase for subjects who completed Part A to accumulate at least 100 EDs; and Part B, a surgical phase.
Part A of the study evaluated the PK (single dose of 60 IU/kg), safety and efficacy of Jivi for on-demand treatment and routine prophylaxis (see Table 5). A total of 134 PTPs (12 to 65 years of age) received at least one infusion of Jivi, including 13 subjects ages 12 to 17 years. One hundred thirty two subjects were evaluable for efficacy, of which 126 (94%) subjects (prophylaxis group: n=108; on-demand group: n=18) completed the 36 weeks of treatment in Part A. The primary efficacy variable was annualized bleed rate (ABR).
A total of 121 subjects received treatment during the extension phase of Study 1 [107 subjects received prophylaxis; 14 subjects continued episodic (on‑demand) treatment].
Safety and efficacy of Jivi in hemostasis during major surgical procedures were evaluated in Part B. Seventeen subjects participated in Part B of Study 1.
|
||
|
Part A (n=132) |
||
|
On-Demand (n=20) |
Prophylaxis* (n=112) |
|
|
Age: median (years) |
48 |
33 |
|
Previous Factor VIII treatment type: N (% ) On-Demand (episodic) Prophylaxis |
20 (100.0%) 0 (0%) |
23 (20.5%) 89 (79.5%) |
|
# of target joints at baseline (mean ± SD) |
2.5 ± 2.1 |
1.5 ± 1.5 |
|
Joint hemorrhage history (mean ± SD of joint bleeds during 12 months prior study) |
23.6 ± 18.8 |
9.5 ± 15.2 |
|
Total treatment duration (Main efficacy period) |
36 weeks |
26 weeks† |
|
ITT population for main efficacy analysis |
20 |
110b |
On-demand Treatment and Control of Bleeding Episodes
In Part A (Weeks 0 – 36), a total of 388 bleeding episodes were treated with Jivi in the on-demand group; 317 bleeding episodes were treated in the prophylaxis groups (see Table 6 below). During the extension phase, 14 subjects receiving on-demand treatment and 107 subjects on routine prophylaxis had 514 and 428 total bleeds, respectively at the cut-off date for the interim analysis.
Approximately 90% of the bleeds were successfully treated with 1 or 2 infusions in both the on-demand and prophylaxis groups (see Table 6). The response to treatment was similar in the extension phase.
|
Characteristics of Bleeding Episodes |
Part A |
|
|
On Demand n=20 |
Total Prophylaxis n=112 |
|
|
Total number of bleeds treated |
388a |
317 a |
|
309 (80%) |
263 (83%) |
|
45 (12%) |
22 (7%) |
|
34 (8.8%) |
32 (10.1%) |
|
Number of bleeds with assessment |
384 |
310 |
|
Number of responses to treatment of bleeds assessed as ‘Excellent’ or ‘Good’ (%) |
253 (66%) |
256 (83%) |
|
Number of responses to treatment of bleeds assessed as ‘Excellent’ or ‘Good’ (%) |
509 (73.3%) |
|
|
Number of responses to treatment of bleeds assessed as ‘Moderate’ |
162 (23.3%) |
|
|
Number of responses to treatment of bleeds assessed as ‘Poor’ |
23 (3.3%) |
|
- Definitions:
- Excellent: Abrupt pain relief and/or improvement in signs of bleeding with no additional infusion administered
- Good: Definite pain relief and/or improvement in signs of bleeding, but possibly requiring more than one infusion for complete resolution
- Moderate: Probable or slight improvement, with at least one additional infusion for complete resolution
- Poor: No improvement or condition worsened
- aFor two bleeds in the on-demand group and one bleed in the prophylaxis group, limited information is available.
Perioperative Management
A total of 17 subjects successfully completed 20 major surgeries in Part B of Study 1 (14 subjects with 17 surgeries) or the extension study (3 subjects with 3 surgeries), using Jivi for hemostasis. There were 6 non-orthopedic surgeries and 14 orthopedic surgeries (3 arthroplasties, 6 joint replacements, 3 synovectomies, and 2 other joint procedures). Treatment with Jivi provided ‘good’ or ‘excellent’ hemostatic control during all 20 major surgeries. The initial Jivi pre-surgery doses administered ranged between 2500 and 5000 IU. The median total dose per surgery was 219 IU/kg with a median of 35 IU/kg/infusion and a median of 7 infusions per surgery (up to 3 weeks). The median number of infusions on day of surgery was 2 (range 1 – 3).
An additional 17 minor surgeries were performed in 10 subjects during Part A of Study 1. The adequacy of hemostasis during minor surgeries was assessed as either ‘good’ or ‘excellent’ in all reported cases.
Routine Prophylaxis
In Study 1, the primary assessment of efficacy was based on 110 subjects who received Jivi for routine prophylaxis during Weeks 10–36 of Part A. Of these, 107 subjects participated in the optional extension phase.
All (n=110) subjects in the prophylaxis treatment arms began treatment with twice weekly infusions of 25 IU/kg for 10 weeks (run-in phase). After the run-in phase (Weeks 0 – 10), subjects (97 of 110; 88%) who experienced ≤ 1 breakthrough bleeds during the first 10 weeks of treatment qualified for randomization to a less frequent dosing regimen, 86 were randomized 1:1 (n = 43 to either arm) to either every 5 days (45–60 IU/kg) or every 7 days for an additional 26 weeks (Weeks 10–36; 6.5 months) (See Table 7). Dose adjustments were recommended for randomized subjects who experienced 2 joint and/or muscle bleeds within a 10-week interval during week 10-36 and included the increase of the dose up to 60 IU/kg or changing to more frequent dosing.
Twelve percent of subjects (n=13) who experienced ≥ 2 spontaneous bleeds during the 10 week run-in phase were ineligible for randomization and continued on the 2 times per week dosing frequency at a higher dose (30–40 IU/kg) for the additional 26 weeks. Nine of the thirteen subjects were on prior prophylaxis (n=9) and were observed to have a higher mean number of bleeds in the 12 months prior to study entry of 17.4 compared to a mean of 5–7 bleeds for all other patients eligible for randomization to less frequent dosing regimens. The median cumulative number of days in the study (Part A plus extension) was 716 (range: 0 to 952 days) with a median number of 137 EDs (range: 1 to 309 EDs). Prophylaxis dose per treatment regimen is summarized in Table 7.
In Weeks 10–36 of Part A, the majority [99/110 (90%)] of subjects did not change their treatment regimens. All subjects randomized to the every 5-day regimen (43/43 subjects) or assigned to the 2 times per week regimen (24/24 subjects) remained in their assigned treatment arm until Week 36. Treatment success in the every 7 day arm was not established. For ABR by regimen, see Table 8. During the extension phase of Study 1, the median prophylaxis dose was maintained for the median duration of 1.3 years (range 0.1–1.9 years).
|
Weeks 10–36*of Part A |
|||
|
Subjects per regimen (n) |
2 times per week 30–40 IU/kg |
Every 5 days (45–60 IU/kg) |
|
|
Eligible for randomization† |
Ineligible for randomization‡ |
||
|
n=11 |
n=13 |
n=43 |
|
|
Median prophylaxis dose/infusion (range) |
30.6 IU/kg (29–41 IU/kg) |
39.2 IU/kg (33–42 IU/kg) |
45.3 IU/kg (39–58 IU/kg) |
An analysis compared ABRs between the on-demand group and the different prophylaxis regimens indicated that the ABR was significantly reduced by 88.2% in the every 5-days (p<0.0001) in comparison with on-demand treatment. There was no significant difference in ABRs between the twice weekly and extended interval treatment arms. Nineteen (19) of 43 subjects in the every 5-day arm (44%) experienced no bleeding episode during week 10–36.
| Main Study (Week 10 – 36) | ||||||
|---|---|---|---|---|---|---|
| Treatment Regimen (n) | Type of Bleed | Subjects with Zero Bleeds, % (n) | ||||
| Total | Spontaneous | Joint | ||||
|
2 times per week 30–40 IU/kg |
Eligible for randomization (11) |
Median (Q1; Q3) |
1.9 (0.0; 5.2) |
0.0 (0.0; 1.9) |
1.9 (0.0; 5.2) |
46% (5) |
|
Mean (SD) |
2.2 (2.7) |
1.2 (2.2) |
2.2 (2.7) |
|||
|
Ineligible for randomization (13) |
Median (Q1; Q3) |
4.1 (2.0; 10.6) |
3.9 (0.0; 4.1) |
4.0 (2; 8.0) |
15% (2) |
|
|
Mean (SD) |
7.2 (7.5) |
3.9 (4.3) |
5.2 (4.8) |
|||
|
Every 5 days 45 – 60 IU/kg (43) |
Median (Q1; Q3) |
1.9 (0.0; 4.2) |
0.0 (0.0; 4) |
1.9 (0.0; 4) |
44% (19) |
|
|
Mean (SD) |
3.3 (4.3) |
1.8 (2.6) |
2.5 (3.5) |
|||
|
On-Demand2 (20) |
Median (Q1; Q3) |
24.1 (17.8; 37.3) |
14.3 (7.3; 22.7) |
16.3 (11.6; 30.3) |
0 (0) |
|
|
Mean (SD) |
28.8 (17.8) |
17.2 (13.2) |
22.2 (16.7) |
|||
1 The ABR was calculated based on the time treated in the assigned treatment regimen.
2 The treatment period for on-demand was Weeks 0 – 36.
15 REFERENCES
1. Church N, Leong L, Katterle Y, et al. Factor VIII activity of BAY 94-9027 is accurately measured with most commonly used assays: results from an international laboratory study. Haemophilia. 2018;00:1-10. http://doi.org/10.1111/hae.13564
2. Coyle TE, Reding MT, Lin JC, Michaels LA, Shah A, Powell J. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. Journal of Thrombosis and Haemostasis. 2014;12(4):488-496. doi:10.1111/jth.12506.
3. Reding MT, Ng HJ, Poulsen LH, et al. Safety and efficacy of BAY 94‐9027, a prolonged‐half‐life factor VIII. Journal of Thrombosis and Haemostasis. 2017;15:411‐419.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
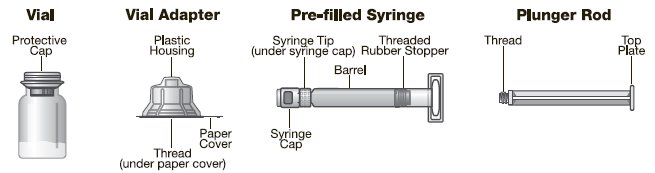
Jivi is available as a lyophilized powder in single-use glass vials, one vial per carton. It is supplied with a sterile vial adapter with 15-micrometer filter and a prefilled diluent glass barrel syringe, which together serve as a needleless reconstitution system. The prefilled diluent syringe contains Sterile Water for Injection, USP. An administration set is also provided in the package. Available sizes:
|
Nominal Strength (IU) |
Diluent (mL) |
Kit NDC Number |
Color Code |
|
500 |
2.5 |
0026-3942-25 |
Green |
|
1000 |
2.5 |
0026-3944-25 |
Red |
|
2000 |
2.5 |
0026-3946-25 |
Yellow |
|
3000 |
2.5 |
0026-3948-25 |
Gray |
Actual Factor VIII activity in IU is stated on the label of each Jivi vial.
The product vial and diluent syringe are not made with natural rubber latex.
Storage and Handling
Product as Packaged for Sale
- •
- Store Jivi at +2°C to +8°C (36°F to 46°F) for up to 24 months from the date of manufacture. Do not freeze. Within this period, Jivi may be stored for a single period of up to 6 months at temperatures up to +25°C or 77°F.
- •
- Record the starting date of room temperature storage on the unopened product carton. Once stored at room temperature, do not return the product to the refrigerator. The shelf-life then expires after storage at room temperature for 6 months, or after the expiration date on the product vial, whichever is earlier.
- •
- Do not use Jivi after the expiration date indicated on the vial.
- •
- Protect Jivi from extreme exposure to light and store the vial with the lyophilized powder in the carton prior to use.
Product After Reconstitution
- •
- Administer reconstituted Jivi as soon as possible. If you do not administer the reconstituted Jivi immediately, then store at room temperature for no longer than 3 hours.
- •
- Do not use Jivi if the reconstituted solution is cloudy or has particulate matter.
- •
- Use the administration set provided.
17 PATIENT COUNSELING INFORMATION
- •
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Hypersensitivity reactions are possible with Jivi [see Warnings and Precautions (5.1)]. Warn patients of the early signs of hypersensitivity reactions (including tightness of the chest or throat, dizziness, mild hypotension and nausea during infusion) which can progress to anaphylaxis. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment with resuscitative measures such as the administration of epinephrine and oxygen.
- •
- Inhibitor formation may occur at any time in the treatment of a patient with hemophilia A [see Warnings and Precautions (5.2)]. Advise patients to contact their physician or treatment center for further treatment and/or assessment, if they experience a lack of clinical response to Factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
- •
- Allergic reactions to polyethylene glycol (PEG), a component of Jivi, are possible. Advise patients to contact their physician or treatment center if they experience a lack of clinical response from their usual dose. [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)]
- •
- Advise patients to discard all equipment, including any unused product, in an appropriate container.
- •
- Advise patients to consult with their healthcare provider prior to travel. Advise patients to bring an adequate supply of Jivi while traveling based on their current regimen of treatment.
Patient Package Insert
FDA-Approved Patient Labeling
Patient Information
Jivi (JIHV-ee)
antihemophilic factor (recombinant), PEGylated-aucl
This leaflet summarizes important information about Jivi with vial adapter. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about Jivi. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to self-infuse, unless your healthcare provider or hemophilia center has taught you how to self-infuse.
What is Jivi?
Jivi is an injectable medicine used to replace clotting factor (Factor VIII or antihemophilic factor) that is missing in people with hemophilia A (congenital Factor VIII deficiency).
Jivi is used to treat and control bleeding in previously treated adults and adolescents (12 years of age and older ) with hemophilia A. Your healthcare provider may also give you Jivi when you have surgery. Jivi can reduce the number of bleeding episodes in adults and adolescents with hemophilia A when used regularly (prophylaxis).
Jivi is not for use in children < 12 years of age or in previously untreated patients.
Jivi is not used to treat von Willebrand disease.
Who should not use Jivi?
You should not use Jivi if you
- •
- are allergic to rodents (like mice and hamsters).
- •
- are allergic to any ingredients in Jivi.
What should I tell my healthcare provider before I use Jivi?
Tell your healthcare provider about:
- •
- All of your medical conditions that you have or had.
- •
- All of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies.
- •
- Pregnancy or planning to become pregnant. It is not known if Jivi may harm your unborn baby.
- •
- Breastfeeding. It is not known if Jivi passes into the milk.
- •
- Whether you have been told that you have inhibitors to Factor VIII.
What are the possible side effects of Jivi?
The common side effects of Jivi are headache, cough, nausea and fever.
Allergic reactions may occur with Jivi. Call your healthcare provider right away and stop treatment if you get tightness of the chest or throat, dizziness, decrease in blood pressure, or nausea. Allergic reactions to polyethylene glycol (PEG), a component of Jivi, are possible.
Your body can also make antibodies, called “inhibitors”, against Jivi, which may stop Jivi from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to Factor VIII.
If your bleeding is not being controlled with your usual dose of Jivi, consult your doctor immediately. You may have developed Factor VIII inhibitors or antibodies to PEG and your doctor may carry out tests to confirm this.
These are not all the possible side effects with Jivi. You can ask your healthcare provider for information that is written for healthcare professionals.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
What are the Jivi dosage strengths?
Jivi with 2.5 mL Sterile Water for Injection (SWFI) comes in four different dosage strengths labeled as International Units (IU): 500 IU, 1000 IU, 2000 IU, and 3000 IU. The four different strengths are color-coded as follows:
|
Green |
500 IU with 2.5 mL SWFI |
|
Red |
1000 IU with 2.5 mL SWFI |
|
Yellow |
2000 IU with 2.5 mL SWFI |
|
Gray |
3000 IU with 2.5 mL SWFI |
How do I store Jivi?
Do not freeze Jivi .
Store Jivi at +2°C to +8°C (36°F to 46°F) for up to 24 months from the date of manufacture. Within this period, Jivi may be stored for a period of up to 6 months at temperatures up to +25°C or 77°F.
Record the starting date of room temperature storage clearly on the unopened product carton. Once stored at room temperature, do not return the product to the refrigerator. The product then expires after storage at room temperature for 6 months, or after the expiration date on the product vial, whichever is earlier. Store vials in their original carton and protect them from extreme exposure to light.
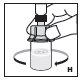
Administer reconstituted Jivi as soon as possible. If not, store at room temperature for no longer than 3 hours.
Throw away any unused Jivi after the expiration date.
Do not use reconstituted Jivi if it is not clear.
What else should I know about Jivi and hemophilia A?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use Jivi for a condition for which it is not prescribed. Do not share Jivi with other people, even if they have the same symptoms that you have.
This leaflet summarizes the most important information about Jivi that was written for healthcare professionals.
Instructions for Use
Jivi-antihemophilic factor (recombinant), PEGylated-aucl
Do not attempt to self-infuse unless you have been taught how by your healthcare provider or hemophilia center.
You should always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using Jivi. If you are unsure of the procedures, please call your healthcare provider before using.
If bleeding is not controlled after using Jivi, then call your healthcare provider right away.
Your healthcare provider will prescribe the dose that you should take.
Your healthcare provider may need to take blood tests from time to time.
Talk to your healthcare provider before traveling. You should plan to bring enough Jivi for your treatment during this time.
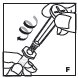
See the step-by-step instructions below for reconstituting (mixing) Jivi with vial adapter. Follow the specific infusion instruction leaflet included with the infusion set provided.
Carefully handle Jivi. Dispose of all materials, including any leftover reconstituted Jivi product, in an appropriate container.
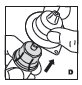
Use only the components for reconstitution and administration that are provided with each package of Jivi. If a package is opened or damaged, do not use this component. If these components cannot be used, please contact your healthcare provider. Gather all the materials needed for the infusion.
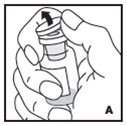
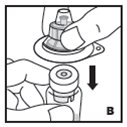
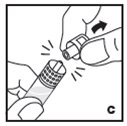
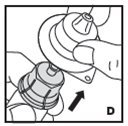
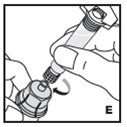
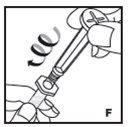
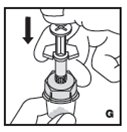
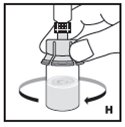
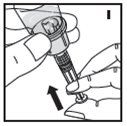
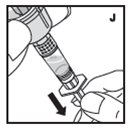
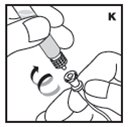
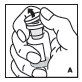
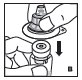
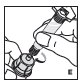
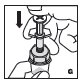
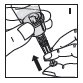
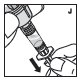
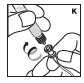
Reconstitution
Always work on a clean flat surface and wash your hands before performing the following procedures.
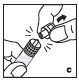
Rate of Administration
The entire dose of Jivi can usually be infused within 1 to 15 minutes. The maximum rate is 2.5 mL per minute. Your healthcare provider will determine the rate of administration that is best for you.
Resources at Bayer available to the patient:
For Adverse Reaction Reporting, contact Bayer Medical Communications 1-888-84-BAYER (1-888-842-2937)
To receive more product information, contact Jivi Customer Service 1-888-606-3780
Bayer Reimbursement HELPline 1-800-288-8374
For more information, visit http://www.Jivi.com
Bayer HealthCare LLC
Whippany, NJ 07981 USA
U.S. License No. 0008
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0026-3942-25
Jivi
500 IU Range
Antihemophilic Factor (Recombinant)
PEGylated-aucl
Recombinant Factor VIII
with Vial Adapter
For Intravenous Use Only
Needleless Reconstitution Set
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0026-3944-25
Jivi
1000 IU Range
Antihemophilic Factor (Recombinant)
PEGylated-aucl
Recombinant Factor VIII
with Vial Adapter
For Intravenous Use Only
Needleless Reconstitution Set