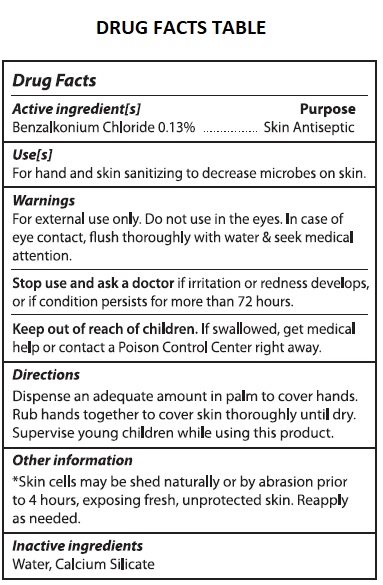
Warnings
For external use only. Do not use in the eyes. In case of eye contact, flush thoroughly with water & seek medical attention.
Stop use and ask a doctor if irritation or redness develops, or if condition persists for more than 72 hours.
Directions
Dispense an adequate amount in palm to cover hands.
Rub hands together to cover skin thoroughly until dry.
Supervise young children while using this product.
Other information
*Skin cells may be shed naturally or by abrasion prior to 4 hours, exposing fresh, unprotected skin. Reapply as needed. Do not allow product to freeze.
Kills 99.99% of Bacteria & Viruses
By DevbondUSA
4-HOUR HAND SANITIZER
NON-FLAMMABLE
NON-TOXIC
DYE FREE
FRAGRANCE FREE
UltraNanoClean Hand Sanitizer has proven residual antimicrobial benefits for up to four hours. It's active ingredient is a preferred skin antiseptic that kills germs on contacts and inhibits future microbial growth. In addition, the proprietary mineral blend enhances product bonding and long lasting effectiveness.
• Kills 99.9% of illness-causing germs on contact & persists on skin for up to 4 hours
• Alcohol Free, Foaming Formula won't dry hands, sting or cause cracking
• Moisture-infused foam leaves skin feeling nourished & silky, even after repeated applications
• Outperforms alcohol-based sanitizers by persisting on skin; guarding against exposure
MADE IN THE USA
Distributed by:
DevBond USA
1836 Augusta Drive
Suite 16
Houston TX 77057
(713) 256-6160
FOR EXTERNAL USE ONLY
FDA Registered