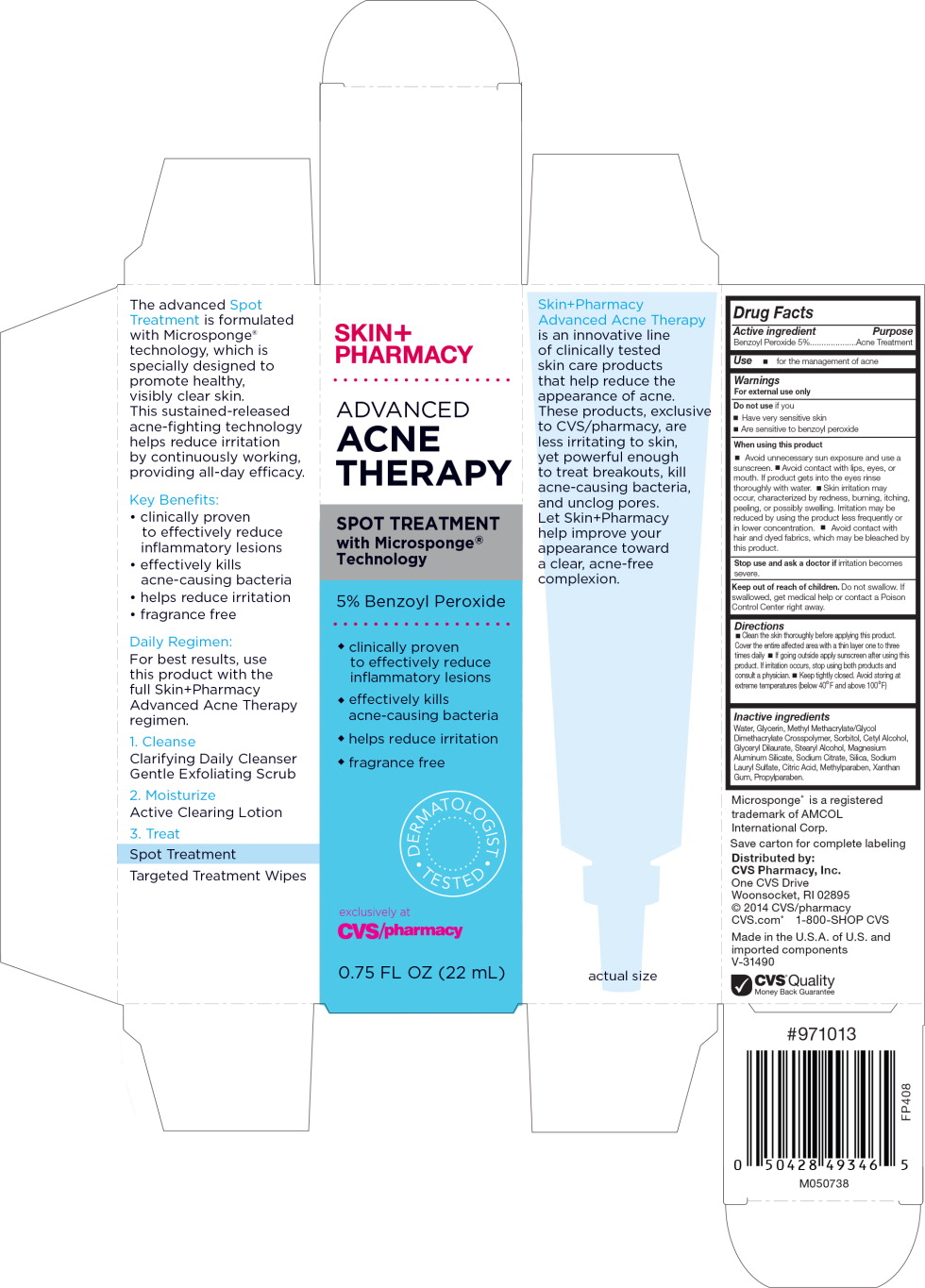
Warnings
For external use only
When using this product
- Avoid unnecessary sun exposure and use a sunscreen.
- Avoid contact with lips, eyes, or mouth. If product gets into the rinse thoroughly with water.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possible swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
- Avoid contact with hair and dyed fabrics, which may be bleached by this product.
Directions
- Clean skin thoroughly before applying this product. Cover the entire affected area with a thin layer one to three times daily.
- If going outside apply sunscreen after using this product. If irritation occurs, stop using both products and consult a physician.
- Keep tightly closed Avoid storing at extreme temperature (below 40°F and above 100°F)
Inactive ingredients
Water, Glycerin, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Sorbitol, Cetyl Alcohol, Glyceryl Dilaurate, Stearyl Alcohol, Magnesium Aluminum Silicate, Sodium Citrate, Silica, Sodium Lauryl Sulfate, Citric Acid, Methylparaben, Xanthan Gum, Propylparaben.
Microsponge® is a registered trademark of AMCOL International Corp.
Save carton for complete labeling
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and imported components
V-31490
CVS® Quality
Money Back Guarantee
#971013
M050738
FP408
Principal Display Panel - Carton Label
SKIN+
PHARMACY
ADVANCED
ACNE
THERAPY
SPOT TREATMENT
with Microsponge®
Technology
5% Benzoyl Peroxide
- clinically proven
to effectively reduce
inflammatory lesions - effectively kills
acne-causing bacteria - helps reduce irritation
- fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy
0.75 FL OZ (22 mL)
Principal Display Panel - Label
SKIN+PHARMACY
ADVANCED
ACNE
THERAPY
SPOT TREATMENT
with Microsponge®
Technology
5% Benzoyl Peroxide
- clinically proven
to effectively reduce
inflammatory lesions - effectively kills
acne-causing bacteria - helps reduce irritation
- fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy
0.75 FL OZ (22 mL)