BOXED WARNING
- BOXED WARNING
Cardiovascular Risk
• NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (see WARNINGS).
• Indomethacin is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Gastrointestinal Risk
• NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).
DESCRIPTION
- Indomethacin cannot be considered a simple analgesic and should not be used in conditions other than those recommended under INDICATIONS AND USAGE.
Indomethacin is a non-steroidal anti-inflammatory indole derivative designated chemically as 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid. Indomethacin, USP is practically insoluble in water and sparingly soluble in alcohol. It has a pKa of 4.5 and is stable in neutral or slightly acidic media and decomposes in strong alkali.
The structural formula is:
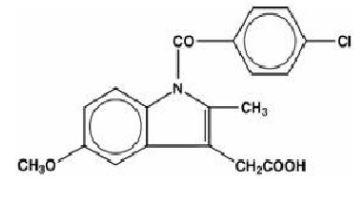
C 19H 16ClNO 4 M.W. 357.80
Each extended-release capsule, for oral administration contains 75 mg of indomethacin and the following inactive ingredients: sugar spheres, povidone, mannitol, isopropyl alcohol, talc. The hard gelatin shell consists of gelatin, iron oxide yellow, titanium dioxide, sodium lauryl sulfate.
The imprinting ink contains the following: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide E172 dye and potassium hydroxide.
This product meets USP Drug Release Test 2 Specifications.
CLINICAL PHARMACOLOGY
-
Indomethacin is a nonsteroidal drug with anti-inflammatory, antipyretic and analgesic properties. Its mode of action, like that of other anti-inflammatory drugs, is not known. However, its therapeutic action is not due to pituitary-adrenal stimulation.
Indomethacin is a potent inhibitor of prostaglandin synthesis in vitro. Concentrations are reached during therapy which have been demonstrated to have an effect in vivo as well. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Moreover, prostaglandins are known to be among the mediators of inflammation. Since indomethacin is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
Indomethacin has been shown to be an effective anti-inflammatory agent, appropriate for long-term use in rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis.
Indomethacin affords relief of symptoms; it does not alter the progressive course of the underlying disease.
Indomethacin suppresses inflammation in rheumatoid arthritis as demonstrated by relief of pain and reduction of fever, swelling and tenderness. Improvement in patients treated with indomethacin for rheumatoid arthritis has been demonstrated by a reduction in joint swelling, average number of joints involved and morning stiffness; by increased mobility as demonstrated by a decrease in walking time; and by improved functional capability as demonstrated by an increase in grip strength.
Indomethacin has been reported to diminish basal and CO2 stimulated cerebral blood flow in healthy volunteers following acute oral and intravenous administration. In one study, after one week of treatment with orally administered indomethacin, this effect on basal cerebral blood flow had disappeared. The clinical significance of this effect has not been established.
Indomethacin extended-release capsules (75 mg) are designed to release 25 mg of drug initially and the remaining 50 mg over approximately 12 hours (90% of dose absorbed by 12 hours). Plasma concentrations of indomethacin fluctuate less and are more sustained following administration of indomethacin extended-release capsules than following administration of 25 mg indomethacin capsules given at 4 to 6 hour intervals. In multiple-dose comparisons, the mean daily steady state plasma level of indomethacin attained with daily administration of indomethacin extended-release capsules 75 mg was indistinguishable from that following indomethacin 25 mg capsules given at 0, 6 and 12 hours daily. However, there was a significant difference in indomethacin plasma levels between the two dosage regimens especially after 12 hours.
Controlled clinical studies of safety and efficacy in patients with osteoarthritis have shown that one capsule of indomethacin extended-release was clinically comparable to one 25 mg indomethacin capsule t.i.d.; and in controlled clinical studies in patients with rheumatoid arthritis, one capsule of indomethacin extended-release taken in the morning and one in the evening were clinically indistinguishable from one 50 mg capsule of indomethacin t.i.d.
Indomethacin is eliminated via renal excretion, metabolism and biliary excretion. Indomethacin undergoes appreciable enterohepatic circulation. The mean half-life of indomethacin is estimated to be about 4.5 hours. With a typical therapeutic regimen of 25 or 50 mg t.i.d., the steady state plasma concentrations of indomethacin are an average 1.4 times those following the first dose.
Indomethacin exists in the plasma as the parent drug and its desmethyl, desbenzoyl and desmethyldesbenzoyl metabolites, all in the unconjugated form. About 60 percent of an oral dosage is recovered in urine as drug and metabolites (26 percent as indomethacin and its glucuronide) and 33 percent is recovered in feces (1.5 percent as indomethacin).
About 99% of indomethacin is bound to protein in plasma over the expected range of therapeutic plasma concentrations. Indomethacin has been found to cross the blood-brain barrier and the placenta.
INDICATIONS & USAGE
-
Carefully consider the potential benefits and risks of indomethacin extended-release capsules and other treatment options before deciding to use indomethacin extended-release capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Indomethacin extended-release capsules have been found effective in active stages of the following:
1. Moderate to severe rheumatoid arthritis including acute flares of chronic disease.
2. Moderate to severe ankylosing spondylitis.
3. Moderate to severe osteoarthritis.
4. Acute painful shoulder (bursitis and/or tendinitis).
Indomethacin extended-release capsules, USP are not recommended for the treatment of acute gouty arthritis.
Indomethacin may enable the reduction of steroid dosage in patients receiving steroids for the more severe forms of rheumatoid arthritis. In such instances the steroid dosage should be reduced slowly and the patients followed very closely for any possible adverse effects.
The use of indomethacin in conjunction with aspirin or other salicylates is not recommended. Controlled clinical studies have shown that the combined use of indomethacin and aspirin does not produce any greater therapeutic effect than the use of indomethacin alone. Furthermore, in one of these clinical studies, the incidence of gastrointestinal side effects was significantly increased with combined therapy. (See PRECAUTIONS,Drug Interactions).
CONTRAINDICATIONS
- Indomethacin extended-release capsules are contraindicated in patients with known hypersensitivity to indomethacin.
Indomethacin extended-release capsules should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS: Anaphylactoid Reactions, and Precautions: Preexisting Asthma).
Indomethacin extended-release capsules is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Warnings
- Cardiovascular Effects
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with a NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and a NSAID does increase the risk of serious GI events (see WARNINGS , Gastrointestinal Effects).
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs, including indomethacin extended-release capsules, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including indomethacin extended-release capsules, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs. Indomethacin extended-release capsules should be used with caution in patients with fluid retention or heart failure
Gastrointestinal EffectsRisk of Ulceration,Bleeding and Perforation
NSAIDs, including indomethacin extended-release capsules, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3 to 6 months, and in about 2 to 4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status.
Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate over renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of indomethacin extended-release capsules in patients with advanced renal disease. Therefore, treatment with indomethacin extended-release capsules is not recommended in these patients with advanced renal disease. If indomethacin extended-release capsules therapy must be initiated, close monitoring of the patient's renal function is advisable.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to indomethacin extended-release capsules. Indomethacin extended-release capsules should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS::(PreexistingAsthma ).Emergency help should be sought in cases where an anaphylactoid reaction occurs. Skin ReactionsNSAIDs, including indomethacin extended-release capsules, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Pregnancy
In late pregnancy, as with other NSAIDs, indomethacin extended-release capsules should be avoided because it may cause premature closure of the ductus arteriosus.
Precautions
- General Precautions
Indomethacin extended-release capsules cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of indomethacin extended-release capsules in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hepatic EffectsBorderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including indomethacin extended-release capsules. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test values has occured, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with indomethacin extended-release capsules. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), indomethacin extended-release capsules should be discontinued.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including indomethacin extended-release capsules. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including indomethacin extended-release capsules, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving indomethacin extended-release capsules who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, indomethacin extended-release capsules should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.Information for PatientsPatients should be informed of the following information before initiating therapy with a NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
1. Indomethacin extended-release capsules, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS : Cardiovascular Effects).
2. Indomethacin extended-release capsules, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects, Risk of Ulceration, Bleeding, and Perforation).
3. Indomethacin extended-release capsules, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
4. Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
5. Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
6. Patients should be informed of the signs of an anaphylactoid reaction (e.g. difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).7. In late pregnancy, as with other NSAIDs, indomethacin extended-release capsules should be avoided because it will cause premature closure of the ductus arteriosus.
Laboratory TestsBecause serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, indomethacin extended-release capsules should be discontinued.
Drug Interactions
Interactions
ACE inhibitors
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Aspirin
When indomethacin extended-release capsules are administered with aspirin, its protein binding is reduced, although the clearance of free indomethacin extended-release capsules is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of indomethacin and aspirin is not generally recommended because of the potential of increased adverse effects.
Furosemide
Clinical studies, as well as post marketing observations, have shown that indomethacin extended-release capsules can reduce the natriuretic effect-of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS, Renal Effects), as well as to assure diuretic efficacy.
Lithium
NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be carefully observed for signs of lithium toxicity.
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Warfarin
The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.Drug & OR Laboratory Test Interactions
Only if positive interactions have been observed. (See 201.57 (f)(4)(N))Carcinogenesis & Mutagenesis & Impairment Of Fertility
Usually only if significant findings have been observed. (See 201.57 (f)(5))Pregnancy
Pregnancy Teratogenic Effects. Pregnancy Category C
Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women.
Nonteratogenic Effects
Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.Labor & Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition and decreased pup survival occurred. The effects of indomethacin extended-release capsules on labor and delivery in pregnant women are unknown.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human-milk and because of the potential for serious adverse reactions in nursing infants from indomethacin extended-release capsules, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Pediatric Use
Safety and effectiveness in pediatric patients below the age of 14 years old have not been established.Geriatric Use
As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older).
Adverse Reactions
- The adverse reactions for indomethacin capsules listed in the following table have been arranged into two groups: (1) incidence greater than 1%; and (2) incidence less than 1%. The incidence for group (1) was obtained from 33 double-blind controlled clinical trials reported in the literature (1,092 patients). The incidence for group (2) was based on reports in clinical trials, in the literature, and on voluntary reports since marketing. The probability of a causal relationship exists between indomethacin and these adverse reactions, some of which have been reported only rarely.
In controlled clinical trials, the incidence of adverse reactions to indomethacin extended-release capsules and equal 24-hour doses of indomethacin capsules were similar.
Incidence greater than 1%
GASTROINTESTINAL
nausea * with or without vomiting
dyspepsia * (including indigestion, heartburn and epigastric pain)
diarrhea
abdominal distress or pain
constipation
CENTRAL NERVOUS SYSTEM
headache (11.7%)
dizziness *
vertigo
somnolence
depression and fatigue (including malaise and listlessness)
SPECIAL SENSES
tinnitus
CARDIOVASCULAR
none
METABOLIC
none
INTEGUMENTARY
none
HEMATOLOGIC
none
HYPERSENSITIVITY
none
GENITOURINARY
none
MISCELLANEOUS
noneIncidence less than 1%
GASTROINTESTINAL
anorexia
bloating (includes distention)
flatulence
peptic ulcer
gastroenteritis
rectal bleeding
proctitis
single or multiple ulcerations, including perforation and hemorrhage of the esophagus, stomach, duodenum or small and large intestines
Intestinal ulceration associated with stenosis and obstruction
gastrointestinal bleeding without obvious ulcer formation and perforation of preexisting sigmoid lesions (diverticulum, carcinoma, etc.) development of ulcerative colitis and regional ileitis
ulcerative stomatitis
toxic hepatitis and jaundice (some fatal cases have been reported)
CENTRAL NERVOUS SYSTEM
anxiety (includes nervousness)
muscle weakness
involuntary muscle movements
insomnia
muzziness
psychic disturbances including psychotic episodes
mental confusion
drowsiness
light-headedness
syncope
paresthesia
aggravation of epilepsy and parkinsonism
depersonalization
coma
peripheral neuropathy
convulsions
dysarthria
SPECIAL SENSES
ocular-corneal deposits and retinal disturbances, including those of the macula, have been reported in some patients on prolonged therapy with Indomethacin
blurred vision
diplopia
hearing disturbances, deafness
CARDIOVASCULAR
congestive heart failure
hypertension
hypotension
tachycardia
chest pain
arrhythmia; palpitations
METABOLIC
edema
weight gain
fluid retention
flushing or sweating
hyperglycemia
glycosuria
hyperkalemia
INTEGUMENTARY
pruritus
rash; urticaria
petechiae or ecchymosis
exfoliative dermatitis
erythema nodosum
loss of hair
Stevens-Johnson Syndrome
erythema multiforme
toxic epidermal necrolysis
HEMATOLOGIC
leukopenia
bone marrow depression
anemia secondary to obvious or occult gastrointestinal bleeding
aplastic anemia
hemolytic anemia
agranulocytosis
thrombocytopenic purpura
disseminated intravascular coagulation
HYPERSENSITIVITY
acute anaphylaxis
acute respiratory distress
rapid fall in blood pressure resembling a shock-like state
angioedema
dyspnea
asthma
purpura
angiitis
pulmonary edema
fever
GENITOURINARY
hematuria
vaginal bleeding
proteinuria, nephrotic syndrome, interstitial nephritis
BUN elevation
renal insufficiency including renal failure
MISCELLANEOUS
epistaxis
breast changes, including enlargement and tenderness, or gynecomastia*Reactions occurring in 3% to 9% of patients treated with indomethacin. (Those reactions occurring in less than 3% of the patients are unmarked.)
Causal Relationship Unknown: Other reactions have been reported but occurred under circumstances where a causal relationship could not be established. However, in these rarely reported events, the possibility cannot be excluded. Therefore, these observations are being listed to serve as alerting information to physicians:
A rare occurrence of fulminant necrotizing fasciitis, particularly in association with Group A β-hemolytic streptococcus, has been described in persons treated with nonsteroidal anti-inflammatory agents, including indomethacin, sometimes with fatal outcome (see also PRECAUTIONS, General).
Cardiovascular: ThrombophlebitisHematologic: Although there have been several reports of leukemia, the supporting information is weak.
Genitourinary: Urinary frequency
Overdosage
-
The following symptoms may be observed following overdosage: nausea, vomiting, intense headache, dizziness, mental confusion, disorientation, or lethargy. There have been reports of paresthesias, numbness and convulsions.
Treatment is symptomatic and supportive. The stomach should be emptied as quickly as possible if the ingestion is recent. If vomiting has not occurred spontaneously, the patient should be induced to vomit with syrup of ipecac. If the patient is unable to vomit, gastric lavage should be performed. Once the stomach has been emptied, 25 g or 50 g of activated charcoal may be given. Depending on the condition of the patient, close medical observation and nursing care may be required. The patient should be followed for several days because gastrointestinal ulceration and hemorrhage have been reported as adverse reactions of indomethacin. Use of antacids may be helpful.
The oral LD 50 of indomethacin in mice and rats (based on 14 day mortality response) was 50 and 12 mg/kg, respectively.
Dosage and Administration
-
Carefully consider the potential benefits and risks of indomethacin extended-release capsules and other treatment options before deciding to use indomethacin extended-release capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS). Indomethacin extended-release capsules 75 mg are available for oral use. Indomethacin extended-release capsules can be administered once a day and can be substituted for indomethacin 25 mg capsules t.i.d. However, there will be significant differences between the two dosage regimens in indomethacin blood levels, especially after 12 hours (see CLINICAL PHARMACOLOGY). In addition, indomethacin extended-release capsules 75 mg b.i.d. can be substituted for indomethacin 50 mg capsules t.i.d. Indomethacin extended-release capsules may be substituted for all the indications of indomethacin capsules except acute gouty arthritis.
Adverse reactions appear to correlate with the size of the dose of indomethacin in most patients, but not all. Therefore, every effort should be made to determine the smallest effective dosage for the individual patient.
Always give indomethacin extended-release capsules 75 mg with food, immediately after meals or with antacids to reduce gastric irritation.
Pediatric Use: Indomethacin ordinarily should not be prescribed for children 14 years of age and under (see WARNINGS).
Adult Use: Dosage Recommendations for Active Stages of the Following:
1. Moderate to severe rheumatoid arthritis, including acute flares of chronic disease; moderate to severe ankylosing spondylitis; and moderate to severe osteoarthritis.
The following information is provided as background only and refers to immediate-release indomethacin capsules (25 mg or 50 mg):
Suggested Dosage:
The following recommendations on dosing pertain to immediate-release indomethacin capsules, and provide important information regarding the dosage and administration of indomethacin. The prescriber should be aware of this information when considering and prescribing extended-release indomethacin.
Indomethacin capsules 25 mg b.i.d. or t.i.d. If this is well tolerated, increase the daily dosage by 25 or 50 mg, if required by continuing symptoms, at weekly intervals until a satisfactory response is obtained or until a total daily dose of 150 to 200 mg is reached. DOSES ABOVE THIS AMOUNT GENERALLY DO NOT INCREASE THE EFFECTIVENESS OF THE DRUG.
In patients who have persistent night pain and/or morning stiffness, the giving of a large portion, up to a maximum of 100 mg, of the total daily dose at bedtime, either orally or by rectal suppositories, may be helpful in affording relief. The total daily dose should not exceed 200 mg. In acute flares of chronic rheumatoid arthritis, it may be necessary to increase the dosage by 25 mg or, if required, by 50 mg daily.
The following information refers to Extended-release Indomethacin Capsules (75 mg):
If indomethacin extended-release capsules are used for initiating indomethacin treatment, one capsule daily should be the usual starting dose in order to observe patient tolerance since 75 mg per day is the maximum recommended starting dose for indomethacin (see above). If indomethacin extended-release capsules are used to increase the daily dose, patients should be observed for possible signs and symptoms of intolerance since the daily increment will exceed the daily increment recommended for other dosage forms. For patients who require 150 mg of indomethacin per day and have demonstrated acceptable tolerance, indomethacin extended-release capsules 75 mg may be prescribed as one capsule twice daily.
If minor adverse effects develop as the dosage is increased, reduce the dosage rapidly to a tolerated dose and OBSERVE THE PATIENT CLOSELY.
If severe adverse reactions occur, STOP THE DRUG. After the acute phase of the disease is under control, an attempt to reduce the daily dose should be made repeatedly until the patient is receiving the smallest effective dose or the drug is discontinued.
Careful instructions to and observations of, the individual patient are essential to the prevention of serious, irreversible, including fatal, adverse reactions.
As advancing years appear to increase the possibility of adverse reactions, indomethacin extended-release capsules should be used with greater care in the aged.
2. Acute painful shoulder (bursitis and/or tendinitis). Initial Dose: 75 mg to 150 mg daily. When 150 mg is prescribed, give as one capsule twice daily.
The drug should be discontinued after the signs and symptoms of inflammation have been controlled for several days. The usual course of therapy is 7 to 14 days.
How Supplied
-
Indomethacin Extended – release capsules USP 75 mg are size ‘2’ hard gelatin capsules, with dark yellow cap imprinted with ‘H’ and clear transparent body imprinted with ‘105’containing cream spherical pellets. They are supplied as
Bottles of 30 capsules NDC 31722-565-30
Bottles of 60 capsules NDC 31722-565-60
Bottles of 100 capsules NDC 31722-565-01
Bottles of 500 capsules NDC 31722-565-05
Bottles of 1000 capsules NDC 31722-565-10
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture
SPL Medguide
- Indomethacin Extended-release Capsules, USP
MEDICATION GUIDE
for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
( See the end of this Medication Guide for a list of prescription NSAID medicines.)
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death. This chance increases:
• with longer use of NSAID medicines
• in people who have heart disease
NSAID medicines should never be used right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
• can happen without warning symptoms
• may cause death
The chance of a person getting an ulcer or bleeding increases with:
• taking medicines called "corticosteroids" and "anticoagulants"
• longer use
• smoking
• drinking alcohol
• older age
• having poor health
NSAID medicines should only be used:
• exactly as prescribed
• at the lowest dose possible for your treatment
• for the shortest time needed
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
• different types of arthritis
• menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
• if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
• for pain right before or after heart bypass surgery
Tell your healthcare provider:
• about all of your medical conditions.
• about all of the medicines you take. NSAIDs and some other medicines can interact
with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
• if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
• if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)?
Serious side effects include: Other side effects include: · heart attack
· stroke
· high blood pressure
· heart failure from body swelling (fluid retension)
· kidney problems including kidney failure
· bleeding and ulcers in the stomach
· and intestine
· low red blood cells (anemia)
· life-threatening skin reactions
· life-threatening allergic reactions
· liver problems including liver failure
· asthma attacks in people who have asthma· stomach pain
· constipation
· diarrhea
· gas
· heartburn
· nausea
· vomiting
· dizziness
Get emergency help right away if you have any of the following symptoms:
· shortness of breath or trouble breathing
· chest pain
· weakness in one part or side of your body
· slurred speech
· swelling of the face or throat
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
· nausea
· more tired or weaker than usual
· itching
· your skin or eyes look yellow
· stomach pain
· flu-like symptoms
· vomit blood
· there is blood in your bowel movement or it is black and sticky like tar
· unusual weight gain
· skin rash or blisters with fever
· swelling of the arms and legs, hands and feetThese are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)
· Aspirin is an NSAID medicine but it does not increase the chance of a heart attack.Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can alsocause ulcers in the stomach and intestines.
· Some of these NSAID medicines are sold in lower doses without a prescription(over-the-counter). Talk to your healthcare provider before using over-the-counterNSAIDS for more than 10 days.
NSAID medicines that need a prescription
Generic Name
Trade nameCelecoxib Celebrex Diclofenac Cataflam, Voltaren, Arthrotec (combined with misoprostol) Diflunisal Dolobid Etodolac Lodine, Lodine XL Fenoprofen Nalfon, Nalfon 200 Flurbiprofen Ansaid Ibuprofen Motrin, Tab-Profen, Vicoprofen (combined with
hydrocodone), Combunox (combined with oxycodone)Indomethacin Indocin, Indocin SR, Indo-Lemmon, Indomethegan Ketoprofen Oruvail Ketorolac Toradol Mefenamic Acid Ponstel Meloxicam Mobic Nabumetone Relafen Naproxen
Naprosyn, Anaprox, Anaprox DS, EC-Naprosyn, Naprelan, Naprapac (copackaged with lansoprazole)Oxaprozin Daypro Piroxicam Feldene Sulindac Clinoril Tolmetin Tolectin, Tolectin DS, Tolectin 600
This Medication Guide has been approved by the U.S Food and Drug Administration.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured for:
Camber Pharmaceuticals, Inc. 2017702
Piscataway, NJ 08854
By: HETEROTM
Hetero Labs Limited
Jeedimetla, Hyderabad – 500 055, India.