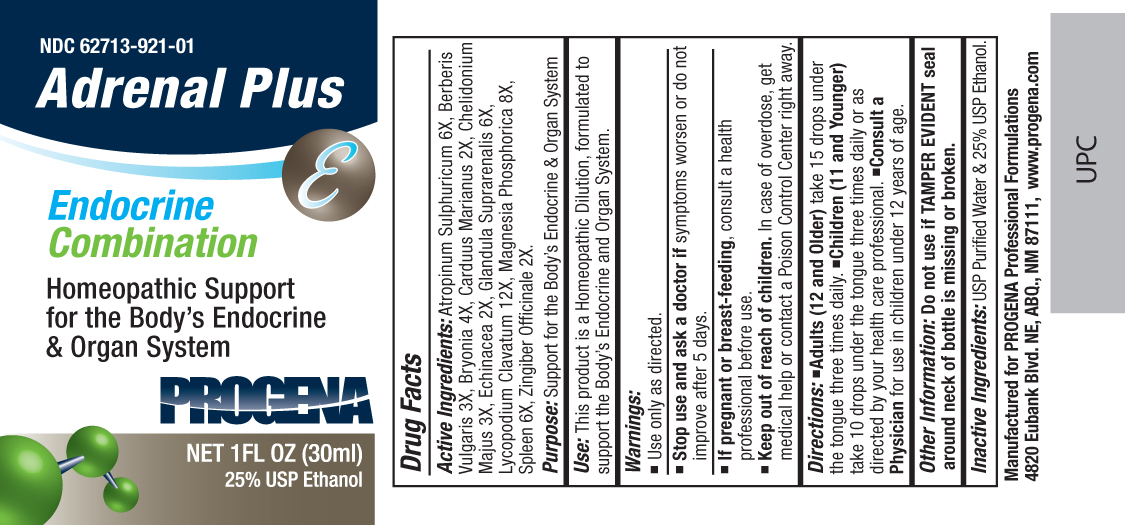
PROGENA ADRENAL PLUS- atropinum sulphuricum, berberis vulgaris, bryonia, carduus marianus, chelidonium majus, echinacea, glandula suprarenalis, lycopodium clavatum, magnesia phosphorica, spleen, zingiber officinale liquid
Meditrend, Inc. DBA Progena Professional Formulations
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients:Atropinum Sulphuricum 6X, Berberis Vulgaris 3X, Bryonia 4X, Carduus Marianus 2X, Chelidonium Majus 3X, Echinacea 2X, Glandula Suprarenalis 6X, Lycopodium Clavatum 12X, Magnesia Phosphorica 8X, Spleen 6X, Zingiber Officinale 2X.
Purpose:Support for the Body's Endocrine and Organ System
Use:This product is a Homeopathic Dilution, formulated to support the Body's Endocrine and Organ System.
Keep Out of Reach of Children. In case of overdose, get medical help or contact a
Poison Control Center right away.
Warnings:
-
Use Only as directed.
-
Stop use and ask a doctor ifsymptoms worsen or do not improve after 5 days.
-
If pregnant or breast-feeding, consult a health professional before use.
Directions:•Adults (12 and Older)take 15 drops under the tongue three times daily.•Children (11 and Younger)take 10 drops under the tongue three times daily or as directed by your health care professional.•Consult a Physicianfor use in children under 12 years of age.
Other Information: Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
Inactive Ingredients:Demineralized water and 25% USP Ethanol.
Manufactured for Progena Professional Formulations
4820 Eubank Blvd. NE, ABQ, NM 87111, www.progena.com
Meditrend, Inc. DBA Progena Professional Formulations