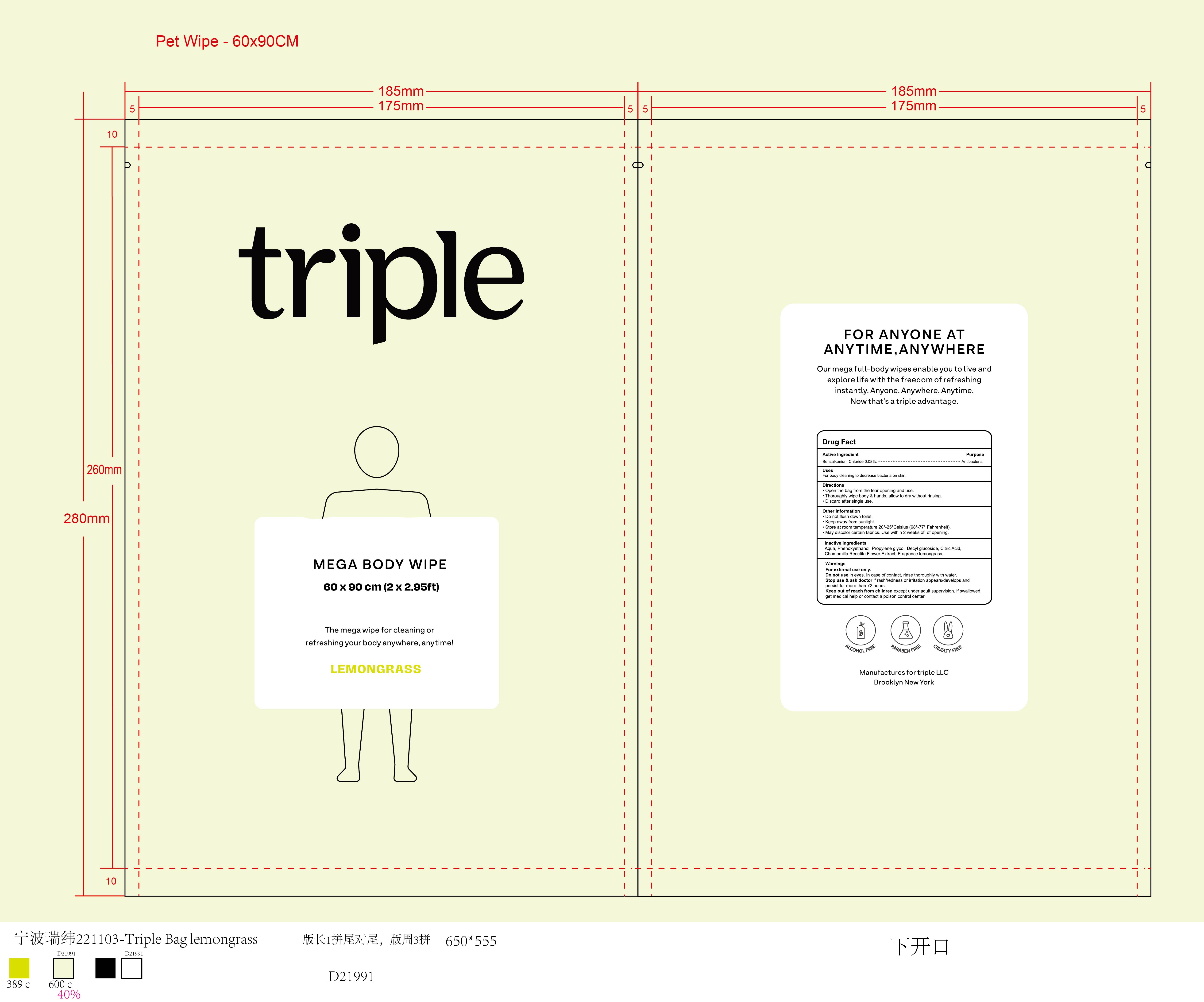
Stop use and ask doctor if rash/redness or irritation appears/develops and persist for more than 72 hours.
Keep out of reach of children except under adult supervision. If swallowed, get medical help or contact a Poison Control Center.
Directions
Open the bag from the tear opening and use.
Thoroughly wipe body & hands, allow to dry without rinsing.
Discard after single use.
Other information
Do not flush down toilet.
Keep away from sunlight.Store at room temperature 20°-25° Celsius(68°-77° Fahrenheit).
May discolor certain fabrics.
Use within 2 weeks of opening.