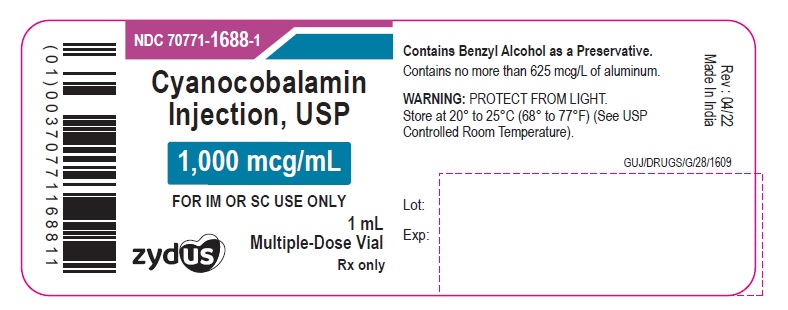
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Cyanocobalamin Injection, USP
1,000 mcg/mL
FOR IM OR SC USE ONLY
1 mL Multiple-dose Vial
Rx only
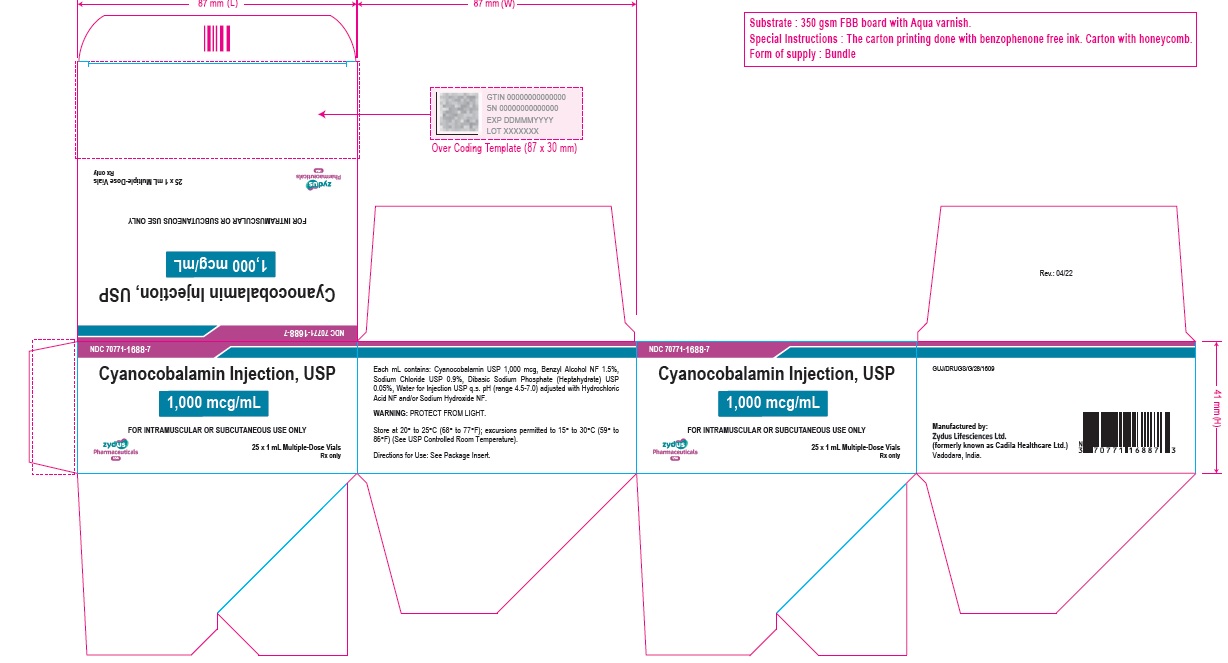
Cyanocobalamin Injection, USP
1,000 mcg/mL
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
25 X 1 mL Multiple-dose Vials
Rx only
Cyanocobalamin Injection, USP
10,000 mcg/10 mL (1,000 mcg/mL)
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
Contains Benzyl Alcohol as a Preservative.
10 mL Multiple-Dose Vial
Rx only

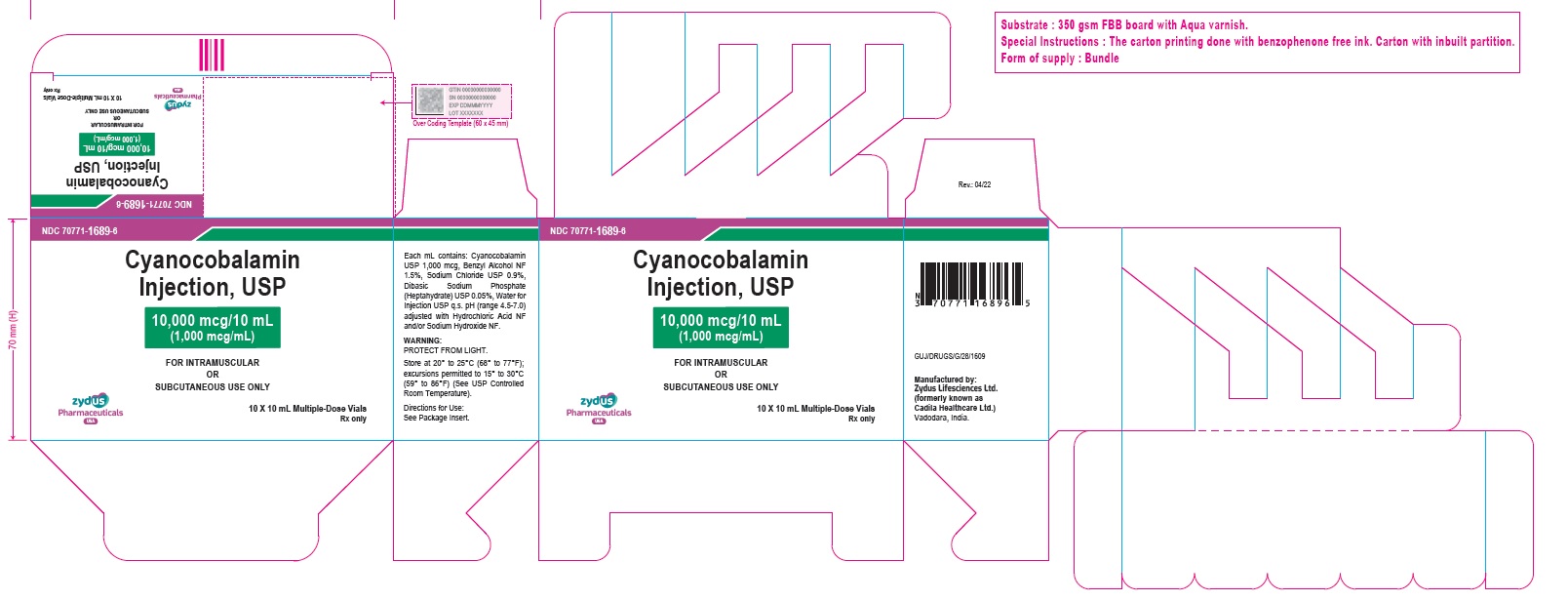
Cyanocobalamin Injection, USP
10,000 mcg/10 mL (1,000 mcg/mL)
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
10 X 10 mL Multiple-Dose Vials
Rx only
Cyanocobalamin Injection, USP
10,000 mcg/10 mL (1,000 mcg/mL)
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
25 X 10 mL Multiple-Dose Vials
Rx only

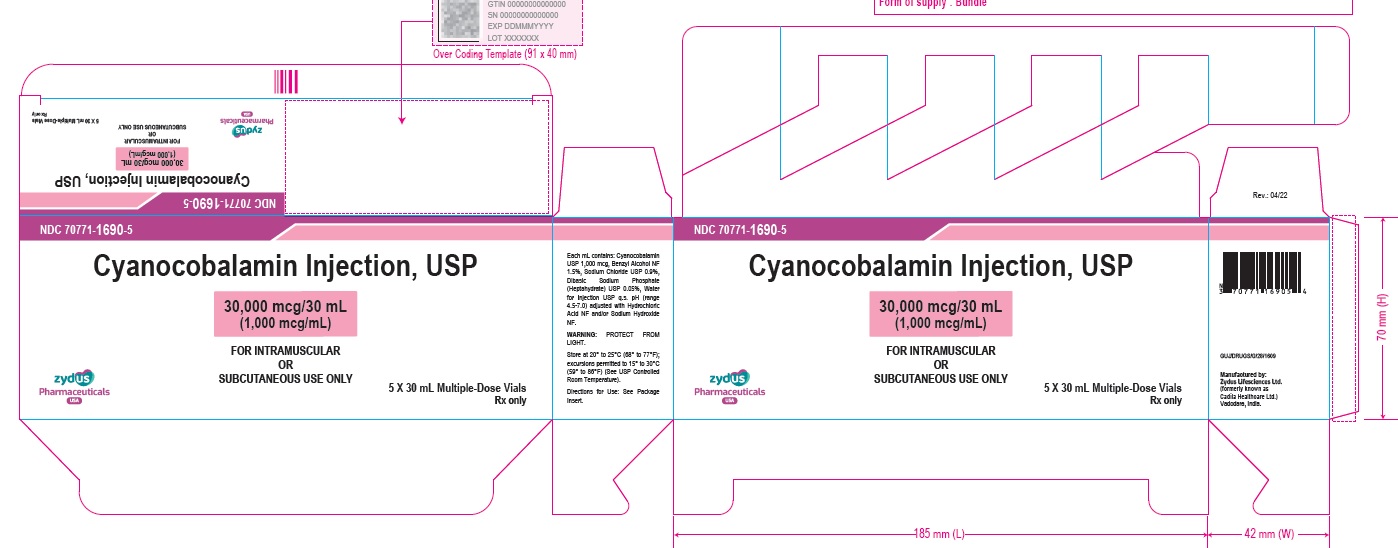
Cyanocobalamin Injection, USP
30,000 mcg/30 mL (1,000 mcg/mL)
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
Contains Benzyl Alcohol as a Preservative.
30 mL Multiple-Dose Vial
Rx only

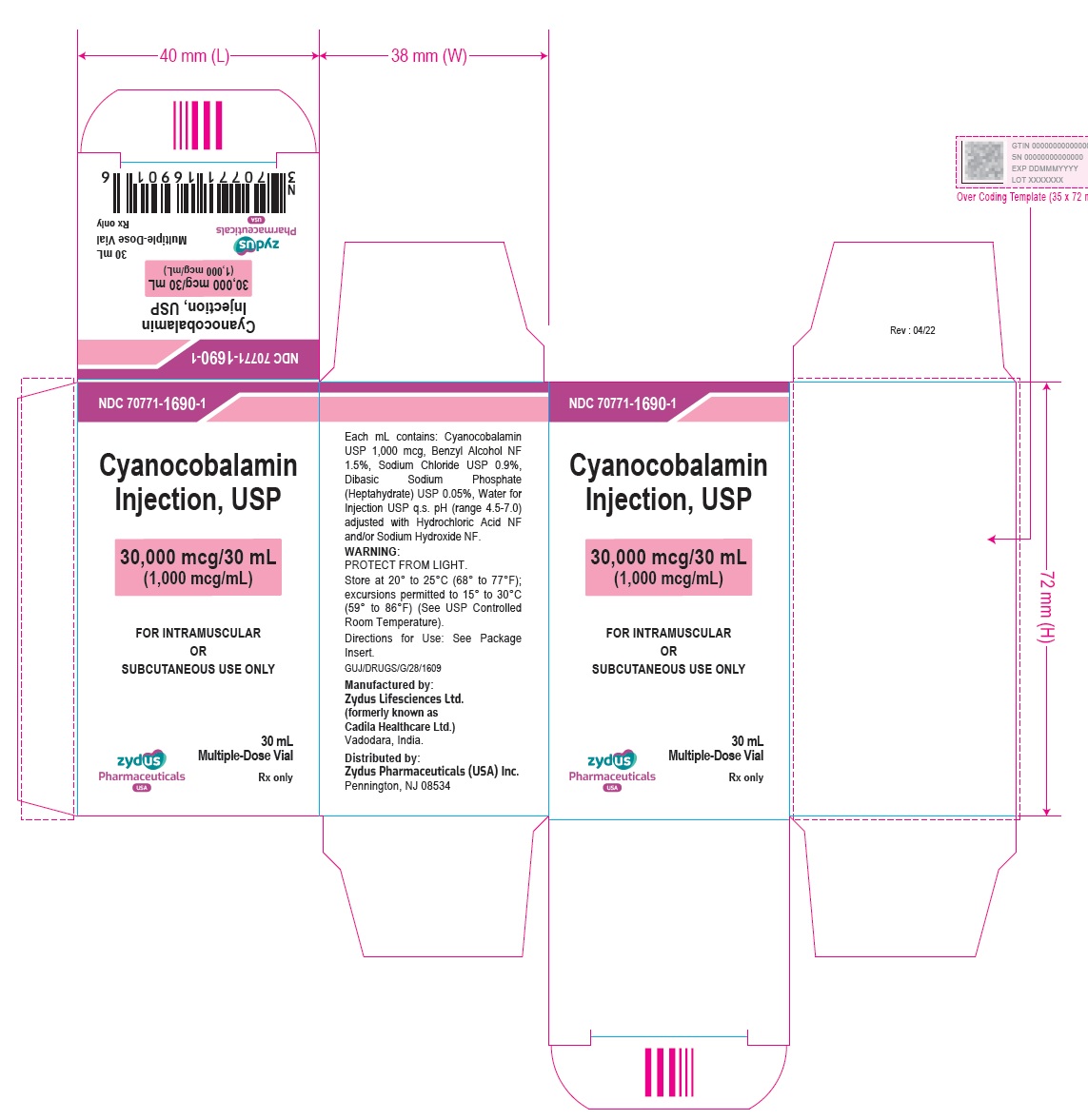
Cyanocobalamin Injection, USP
30,000 mcg/30 mL (1,000 mcg/mL)
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
30 mL Multiple-Dose Vial – Carton Label
Rx only
Cyanocobalamin Injection, USP
30,000 mcg/30 mL (1,000 mcg/mL)
FOR INTRAMUSCULAR OR SUBCUTANEOUS USE ONLY
5 X 30 mL Multiple-Dose Vials
Rx only