Description
Urea 40% is a keratolytic emollient which is a gentle, yet potent, tissue softener for nails and/or skin. Each gram of Urea 40% contains 40% urea as an active ingredient, and the following inactive ingredients: Ceteareth-6, Ceteareth-25, Cetyl Alcohol, Mineral Oil, Propylene Glycol, Purified Water, Sodium Hydroxide, Stearyl Alcohol, Xanthan Gum.
Urea is a diamide of carbonic acid with the following chemical structure:
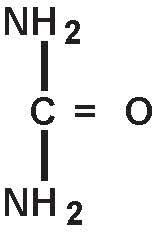
Clinical Pharmacology
Urea gently dissolves the intracellular matrix which results in loosening of the horny layer of the skin and shedding of scaly skin at regular intervals, thereby softening hyperkeratotic areas of the skin.
Indications and Usage
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eshar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.
Precautions
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
PREGNANACY
Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, Urea 40% should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether or not this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Urea 40% is administered to a nursing woman.
Adverse Reactions
Transient stinging, burning, itching or irritation may occur and normally disappear upon discontinuing the medication.
Dosage and Administration
Apply Urea 40% to affected skin twice per day, or as directed by your physician. Rub in until completely absorbed.
Apply to diseased or damaged nail(s) twice per day, or as directed by a physician.
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency.Each person recommending a prescription substitution using this product shall make such recommendations based on each person's professional opinion and knowledge, upon evaluation of the active ingredients, excipients, inactive ingredients and chemical information provided herein.
How Supplied
Urea 40% Cream 1 oz.(28.35 g): NDC 16477-340-01; Urea 40% Cream 3 oz.(85 g): NDC 16477-340-03; Urea 40% Cream 7 oz.(198.4 g): NDC 16477-340-07 Store at room temperature 15°C - 30°C (59°F - 86°F). Protect from freezing. Keep bottle tightly closed.
