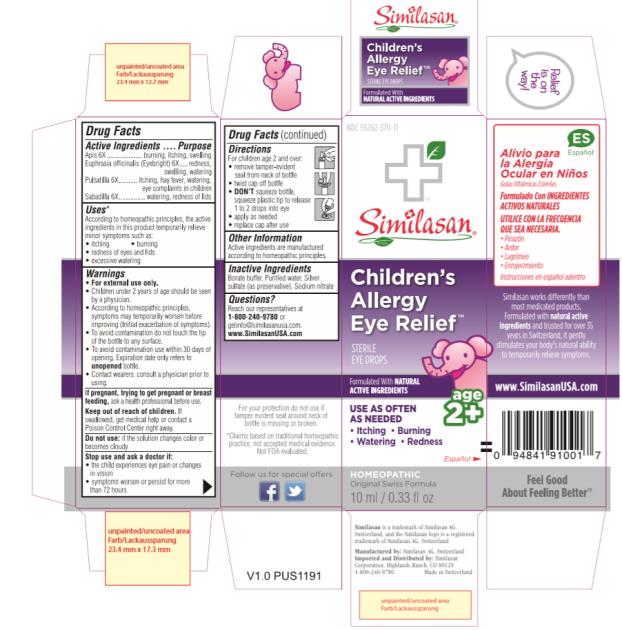
CHILDRENS ALLERGY EYE RELIEF- anemone patens, apis mellifera, euphrasia stricta and schoenocaulon officinale seed solution/ drops
Similasan Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts – Children’s Allergy Eye Relief
Active Ingredients
Apis 6X
Euphrasia officinalis (Eyebright) 6X
Pulsatilla 6X
Sabadilla 6X
Purpose
burning, itching, swelling
redness, swelling, watering
itching, hay fever, watering, eye complaints in children
watering, redness of lids
Uses*
According to homeopathic principles, the active ingredients in this product temporarily relieve minor symptoms such as:
• itching
• burning
• redness of eyes and lids
• excessive watering
Warnings
• For external use only.
• Children under 2 years of age should be seen by a physician.
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
• To avoid contamination do not touch the tip of the bottle to any surface.
• To avoid contamination use within 30 days of opening. Expiration date only refers to unopened bottle.
• Contact wearers: consult a physician prior to using.
If pregnant, trying to get pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Do not use:
if the solution changes color or becomes cloudy
Stop use and ask a doctor if:
• the child experiences eye pain or changes in vision
• symptoms worsen or persist for more than 72 hours
Directions
For children age 2 and over:
• remove tamper-evident seal from neck of bottle
• twist cap off bottle
• DON'T squeeze bottle, squeeze plastic tip to release 1 to 2 drops into eye
• apply as needed
• replace cap after use
Other Information
Active ingredients are manufactured according to homeopathic principles.
Inactive Ingredients
Borate buffer, Purified water, Silver sulfate (as preservative), Sodium nitrate
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com.
www.SimilasanUSA.com
PRINCIPAL DISPLAY PANEL
NDC 59262-370-11
Similasan
Children’s
Allergy
Eye Relief
STERILE EYE DROPS
10 ml / 0.33 fl oz