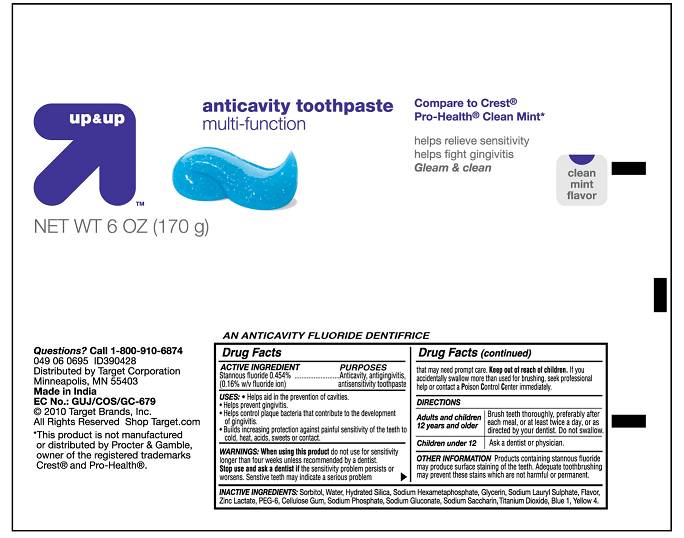
Active Ingredients
STANNOUS FLUORIDE 0.454%...................... ANTICAVITY, ANTIGINGIVITIS,
(0.16% W/V FLUORIDE ION) ANTISENSITIVITY TOOTHPASTE
USES
- HELPS AID IN THE PREVENTION OF CAVITIES
- HELPS PREVENT GINGIVITIS
- HELPS CONTROL PLAQUE BACTERIA THAT CONTRIBUTE TO THE DEVELOPMENT OF GINGIVITIS
- BUILD INCREASING PROTECTION AGAINST PAINFUL SENSITIVITY OF THE TEETH TO COLD, HEAT, ACIDS, SWEETS OR CONTACT.
WARNINGS
WARNINGS: WHEN USING THIS PRODUCT DO NOT USE FOR SENSITIVITY LONGER THAN FOUR WEEKS UNLESS RECOMMENDED BY A DENTIST. STOP USE AND ASK A DENTIST IF THE SENSITIVITY PROBLEM PERSISTS OR WORSENS. SENSITIVE TEETH MAY INDICATE A SERIOUS PROBLEM THAT MAY NEED PROMPT CARE. If you accidentally swallow more than used for brushing, seek professional help or contact a Poison Control Center immediately.Directions
Adults and children 12 years and older Brush teeth thoroughly, PREFERABLY AFTER EACH MEAL,or at least twice a day, or as directed by your dentist. DO NOT SWALLOW
Children under 12 ASK A dentist or PHYSICIAN
Other information PRODUCTS CONTAINING STANNOUS FLUORIDE MAY PRODUCE SURFACE STAINING OF THE TEETH. ADEQUATE TOOTHBRUSHING MAY PREVENT THESE STAIN WHICH ARE NOT HARMFUL OR PERMANENT.