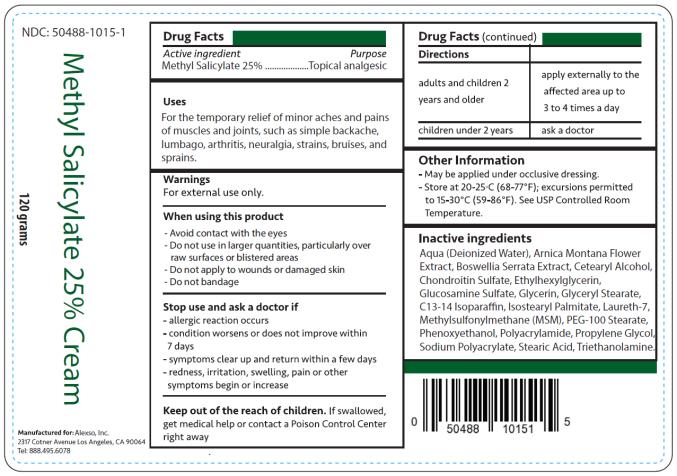
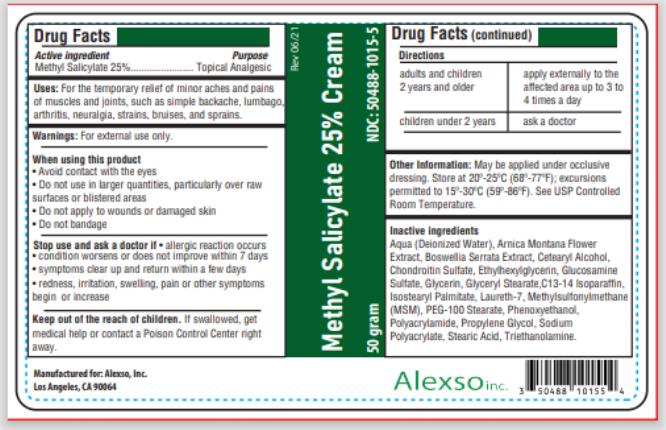
Uses
For the temporary relief of minor aches and pains of muscles and joints, such as simple backache, lumbago, arthritis, neuralgia, strains, bruises, and sprains.
Warnings
For external use only.
When using this product
- Avoid contact with the eyes
- Do not use in large quantities, particularly over raw surfaces or blistered areas
- Do not apply to wounds or damaged skin
- Do not bandage
Directions
| adults and children 2 years and older | apply externally to the affected area up to 3 to 4 times a day |
| children under 2 years | ask a doctor |
Other information
- May be applied under occlusive dressing.
- Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
Inactive ingredients
Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cetearyl Alcohol, Chondroitin Sulfate, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Stearate, C13-14 Isoparaffin, Isostearyl Palmitate, Laureth-7, Methylsulfonylmethane (MSM), PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Propylene Glycol, Sodium Polyacrylate, Stearic Acid, Triethanolamine
Methyl Salicylate 25% Cream
NDC: 50488-1015-5
50 grams
Methyl Salicylate 25% Cream
NDC: 50488-1015-1
120 grams
Manufactured for:
Alexso, Inc
Los Angeles, CA 90064