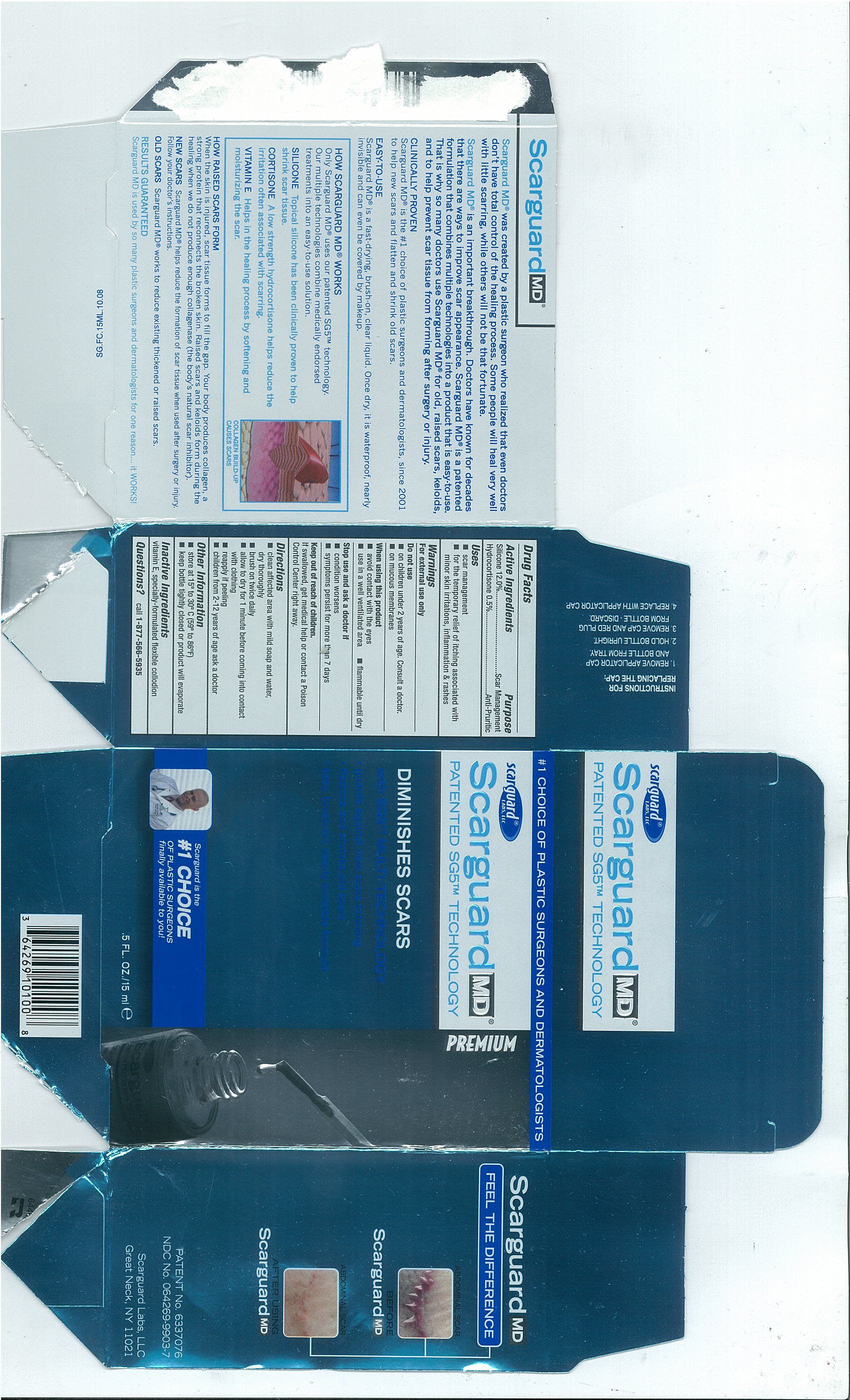
SCARGUARD MD - hydrocortisone silicone liquid
Scarguard Labs, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
- Silicone 12.0%
- Hydrocortisone 0.5%
Purpose
Scar Management
Anti-Pruritic
Uses
- scar management
- for the temporary relief of itching associated with minor skin irritations, inflammation and rashes
Warnings
For external use only
Do Not Use
- on children under 2 years of age. Consult a doctor.
- on mucous membranes
When using this product
- avoid contact with the eyes
- use in a well ventilated area
- flammable until dry
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean affected area with mild soap and water, dry thoroughly
- brush on twice daily
- allow to dry for 1 minute before coming into contact with clothing
- reapply if peeling
- children from 2-12 years of age ask a doctor
Other Information
- store at 15º to 30º C (59º to 86ºF)
- Keep bottle tightly closed or product will evaporate
Inactive Ingredients
vitamin E, specially-formulated flexible collodion
Questions?
call
1-877-566-5935
15 mL carton