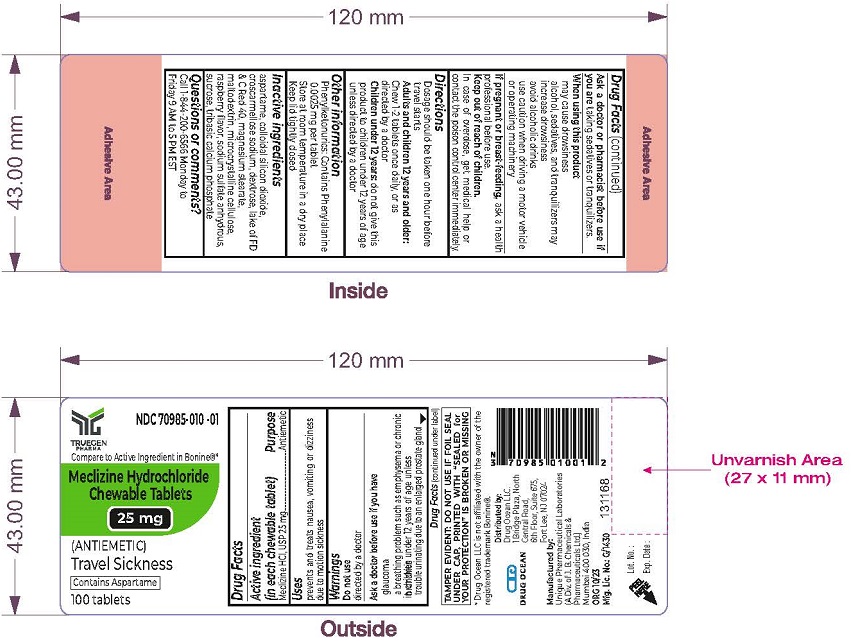
Warnings
Do not use in children under 12 years of age unless directed by a doctor
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
When using this product
- may cause drowsiness
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use caution when driving a motor vehicle or operating machinery
Keep out of reach of children.
In case of overdose, get medical help or contact the poison control center immediately
Directions
- Dosage should be taken one hour before travel starts
- Adults and children 12 years and older: Chew 1-2 tablets once daily, or as directed by a doctor
- Children under 12 years: do not give this product to children under 12 years of age unless directed by a doctor
Other information
- Phenylketonurics: Contains Phenylalanine 0.0025 mg per tablet
- Store at room temperature in a dry place
- Keep lid tightly closed
Inactive ingredients
aspartame, colloidal silicon dioxide, croscarmellose sodium, dextrose, lake of FD & C Red 40, magnesium stearate, maltodextrin, microcrystalline cellulose, raspberry flavor, sodium sulfate anhydrous, sucrose, tribasic calcium phosphate
TAMPER EVIDENT: DO NOT USE IF FOIL SEAL UNDER CAP, PRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING
Distributed by:

Drug Ocean LLC,
1 Bridge Plaza, North Central Road
6th Floor, Suite 675
Fort Lee, NJ 07024
Manufactured by:
Unique Pharmaceutical Laboratories
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd.)
Mumbai 400 030, India
ORG 12/23