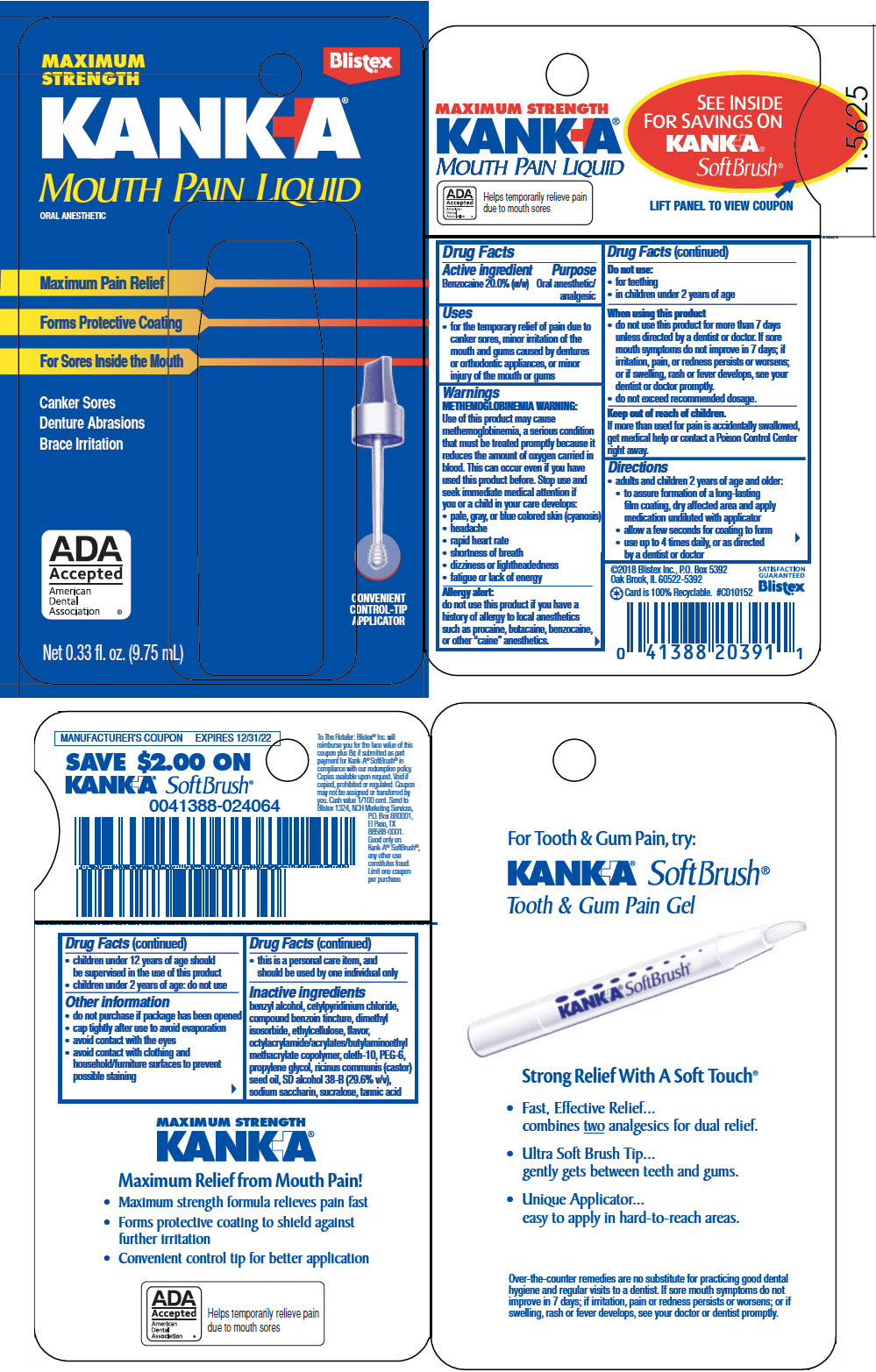
Uses
- for the temporary relief of pain due to canker sores, minor irritation of the mouth and gums caused by dentures or orthodontic appliances, or minor injury of the mouth or gums
Warnings
METHEMOGLOBINEMIA WARNING
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
When using this product
- do not use this product for more than 7 days unless directed by a dentist or doctor. If sore mouth symptoms do not improve in 7 days; if irritation, pain, or redness persists or worsens; or if swelling, rash or fever develops, see your doctor or dentist promptly.
- do not exceed recommended dosage.
Directions
- adults and children 2 years of age and older:
- to assure formation of a long-lasting film coating, dry affected area and apply medication undiluted with applicator
- allow a few seconds for coating to form
- use up to 4 times daily, or as directed by a dentist or doctor
- children under 12 years of age should be supervised in the use of this product
- children under 2 years of age: do not use
Other information
- do not purchase if package has been opened
- cap tightly after use to avoid evaporation
- avoid contact with the eyes
- avoid contact with clothing and household/ furniture surfaces to prevent possible staining
- this is a personal care item, and should be used by one individual only
Inactive ingredients
benzyl alcohol, cetylpyridinium chloride, compound benzoin tincture, dimethyl isosorbide, ethylcellulose, flavor, octylacrylamide/acrylates/butylaminoethyl methacrylate copolymer, oleth-10, PEG-6, propylene glycol, ricinus communis (castor) seed oil, SD alcohol 38B (29.6% v/v), sodium saccharin, sucralose, tannic acid
PRINCIPAL DISPLAY PANEL - 9.75 mL Bottle Package
MAXIMUM
STRENGTH
Blistex®
KANK+A®
MOUTH PAIN LIQUID
ORAL ANESTHETIC
Maximum Pain Relief
Forms Protective Coating
For Sores Inside the Mouth
Canker Sores
Denture Abrasions
Brace Irritation
ADA
Accepted
American
Dental
Association
®
CONVENIENT
CONTROL-TIP
APPLICATOR
Net 0.33 fl. oz. (9.75 mL)