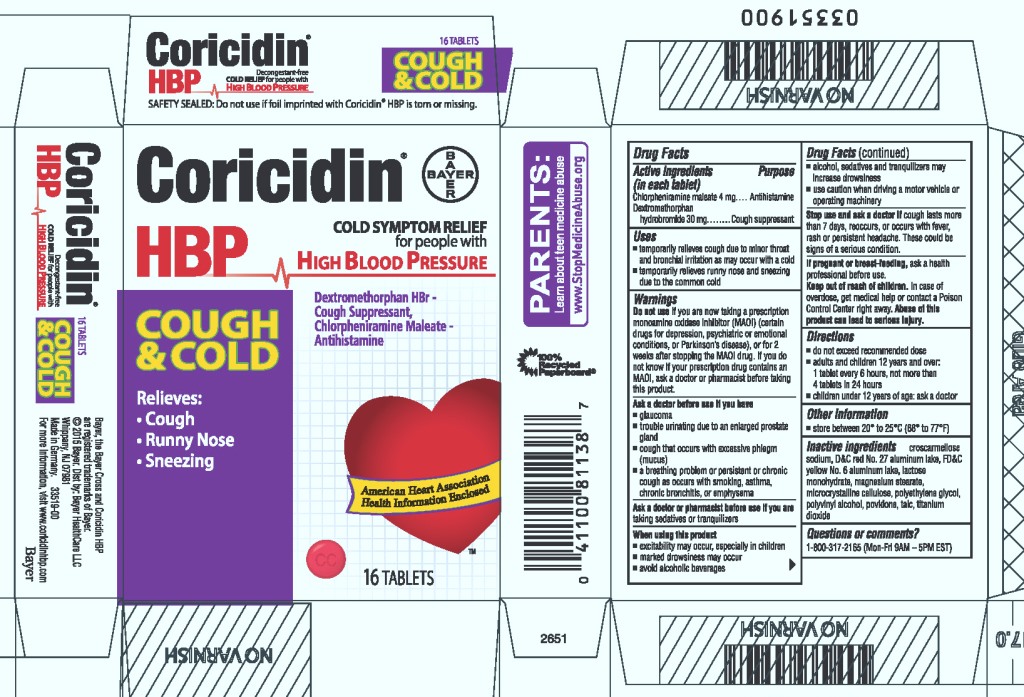
CORICIDIN HBP COUGH AND COLD COUGH SUPPRESSANT, ANTIHISTAMINE- chlorpheniramine maleate and dextromethorphan hydrobromide tablet, film coated
Bayer HealthCare LLC.
----------
| Active ingredients (in each tablet) | Purpose |
| Chlorpheniramine maleate 4 mg | Antihistamine |
| Dextromethorphan hydrobromide 30 mg | Cough suppressant |
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- temporarily relieves runny nose and sneezing due to the common cold
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- cough that occurs with excessive phlegm (mucus)
- a breathing problem or persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if cough lasts more than 7 days, reoccurs, or occurs with fever, rash or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Abuse of this product can lead to serious injury.
Directions
- do not exceed recommended dose
- adults and children 12 years and over:
1 tablet every 6 hours, not more often than
4 tablets in 24 hours
- children under 12 years of age: ask a doctor
Other information
- store between 20° to 25°C (68° to 77°F)
Inactive ingredients
croscarmellose sodium, D&C red No. 27 aluminum lake, FD&C yellow No. 6 aluminum lake, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, talc, titanium dioxide
Questions or comments?
Call 1-800-317-2165 (Mon-Fri 9AM-5PM EST)
PRINCIPAL DISPLAY PANEL - 16 Tablet Carton
Coricidin®
HBP
Decongestant-free
COLD SYMPTOM RELIEF for people with
HIGH BLOOD PRESSURE
Dextromethorphan HBr-Cough Suppressant,
Chlorpheniramine Maleate-Antihistamine
COUGH
&COLD
Relieves:
-
Cough
-
Runny Nose
-
Sneezing
16 TABLETS
Bayer HealthCare LLC.
