DR WALTONS PETROLEUM - petrolatum jelly
Dr. Waltons, Incorporated
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
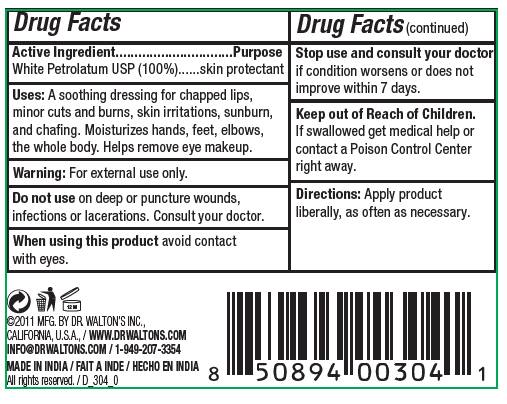
Active Ingredient Purpose
White Petrolatum USP (100%) skin protectant
DIRECTIONS
APPLY PRODUCT LIBERALLY, AS OFTEN AS NECESSARY
DO NOT USE
Do not use on deep or puncture wounds, infections or lacerations. Consult your doctor.
WARNINGS
Warning: For external use only.
KEEP OUT OF REACH OF CHILDREN
Keep out of Reach of Children. If swallowed get medical help or contact a poison control center right away.
PURPOSE
Uses: A soothing dressing for chapped lips, minor cuts and burns, skin irritations, sunburn, and chafing. Moisturizes hands, feet, elbows, the whole body. Helps remove eye makeup.
USES
A SOOTHING DRESSING FOR CHAPPED LIPS, MINOR CUTS AND BURNS, SKIN IRRITATIONS, SUNBURN, AND CHAFING.
 E
E
 nter section text here
nter section text here
Dr. Waltons, Incorporated

