Warnings
If you are pregnant or breast-feeding, only use this medicine on the advice of your health care provider. Smoking can seriously harm your child. Try to stop smoking without using any nicotine replacement medicine. This medicine is believed to be safer than smoking. However, the risks to your child from this medicine are not fully known.
Ask a doctor before use if you have
-
a sodium-restricted diet
-
heart disease, recent heart attack, or irregular heartbeat. Nicotine can increase your heart rate.
-
high blood pressure not controlled with medication. Nicotine can increase blood pressure.
-
stomach ulcer or diabetes
-
history of seizures
Ask a doctor or pharmacist before use if you are
-
using a non-nicotine stop smoking drug
-
taking prescription medicine for depression or asthma. Your prescription dose may need to be adjusted.
Stop use and ask a doctor if
-
mouth, teeth, or jaw problems occur
-
irregular heartbeat or palpitations occur
-
you get symptoms of nicotine overdose such as nausea, vomiting, dizziness, diarrhea, weakness, and rapid heartbeat
-
you have symptoms of an allergic reaction (such as difficulty breathing or rash)
Keep out of reach of children and pets.
Pieces of nicotine gum may have enough nicotine to make children and pets sick. Wrap used pieces of gum in paper and throw away in the trash. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions - 2 mg
-
if you are under 18 years of age, ask a doctor before use
-
before using this product, read the enclosed User's Guide for complete directions and other important information
-
begin using the gum on your quit day
-
if you smoke your first cigarette within 30 minutes of waking up, use 4 mg nicotine gum
-
if you smoke your first cigarette more than 30 minutes after waking up, use 2 mg nicotine gum according to the following 12 week schedule:
|
Weeks 1 to 6 |
Weeks 7 to 9 |
Weeks 10 to 12 |
|
1 piece every |
1 piece every |
1 piece every |
-
nicotine gum is a medicine and must be used a certain way to get the best results
-
chew the gum slowly until it tingles. Then park it between your cheek and gum. When the tingle is gone, begin chewing again until the tingle returns.
-
repeat this process until most of the tingle is gone (about 30 minutes)
-
do not eat or drink for 15 minutes before chewing the nicotine gum, or while chewing a piece
-
to improve your chances of quitting, use at least 9 pieces per day for the first 6 weeks
-
if you experience strong or frequent cravings, you may use a second piece within the hour. However, do not continuously use one piece after another since this may cause you hiccups, heartburn, nausea or other side effects.
-
do not use more than 24 pieces a day
-
it is important to complete treatment. If you feel you need to use the gum for a longer period to keep from smoking, talk to your health care provider.
Directions - 4 mg
- if you are under 18 years of age, ask a doctor before use
-
before using this product, read the enclosed User's Guide for complete directions and other important information
-
begin using the gum on your quit day
-
if you smoke your first cigarette more than 30 minutes after waking up, use 2 mg nicotine gum
-
if you smoke your first cigarette within 30 minutes of waking up, use 4 mg nicotine gum according to the following 12 week schedule:
|
Weeks 1 to 6 |
Weeks 7 to 9 |
Weeks 10 to 12 |
|
1 piece every |
1 piece every |
1 piece every |
-
nicotine gum is a medicine and must be used a certain way to get the best results
-
chew the gum slowly until it tingles. Then park it between your cheek and gum. When the tingle is gone, begin chewing again, until the tingle returns.
-
repeat this process until most of the tingle is gone (about 30 minutes)
-
do not eat or drink for 15 minutes before chewing the nicotine gum, or while chewing a piece
-
to improve your chances of quitting, use at least 9 pieces per day for the first 6 weeks
-
if you experience strong or frequent cravings, you may use a second piece within the hour. However, do not continuously use one piece after another since this may cause you hiccups, heartburn, nausea or other side effects.
-
do not use more than 24 pieces a day
-
it is important to complete treatment. If you feel you need to use the gum for a longer period to keep from smoking, talk to your health care provider.
Other information - 2 mg
• each piece contains: calcium 115 mg, sodium 8 mg
• store at 20 - 25ºC (68 - 77ºF)
• protect from light
Other information - 4 mg
-
each piece contains: calcium 120 mg, sodium 8 mg
-
store at 20 - 25ºC (68 - 77ºF)
-
protect from light
Inactive ingredients - 2 mg
acacia, acesulfame potassium, carnauba wax, flavors, gum base, hydroxypropyl cellulose, magnesium oxide, sodium bicarbonate, sodium carbonate, talc, titanium dioxide and xylitol.
Inactive ingredients - 4 mg
acacia, acesulfame potassium, carnauba wax, D&C yellow #10 aluminum lake, flavors, gum base, hydroxypropyl cellulose, magnesium oxide, sodium bicarbonate, sodium carbonate, talc, titanium dioxide and xylitol.
TO INCREASE YOUR SUCCESS IN QUITTING:
- You must be motivated to quit.
- Use Enough - Chew at least 9 pieces of Nicotine Polacrilex Gum per day during the first six weeks.
- Use Long Enough - Use Nicotine Polacrilex Gum for the full 12 weeks.
- Use with a support program as directed in the enclosed User's Guide.

Instructions
To remove the gum, tear off single unit
Peel off backing starting at corner with loose edge.
Push gum through foil
Blister packaged for your protection.
Do not use if individual seals are open or torn.
- Not for sale to those under 18 years of age.
- Proof of age required.
- Not for sale in vending machines or from any source where proof of age cannot be verified.
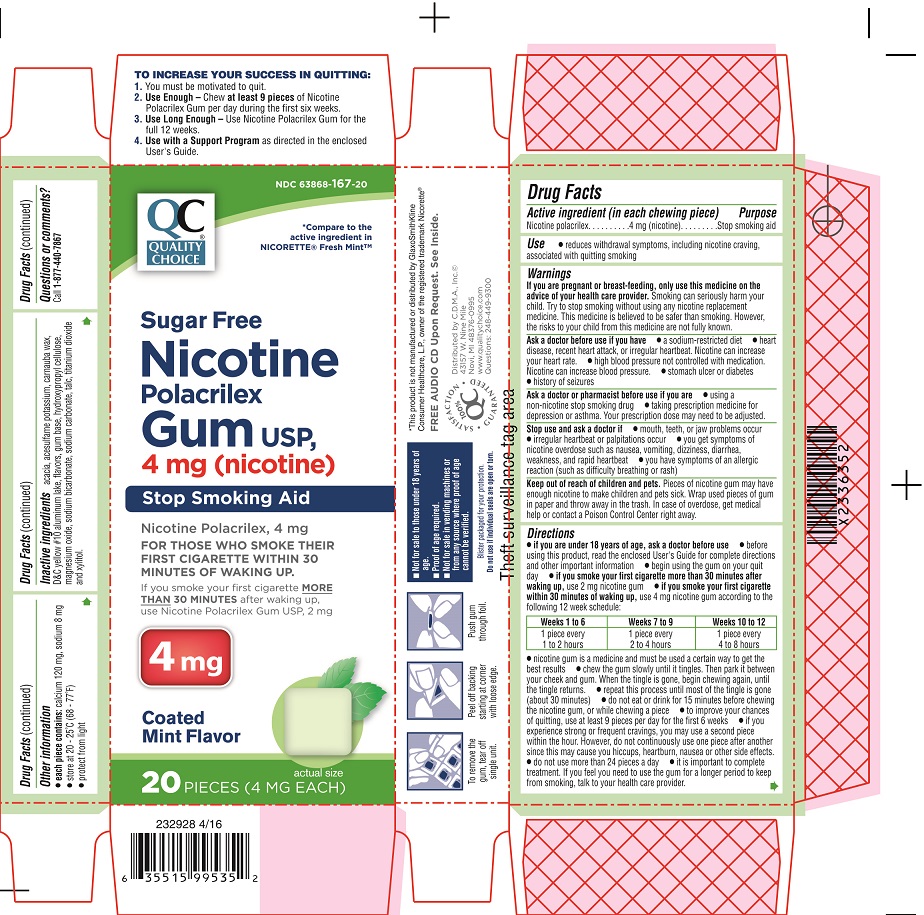
Principal Display Panel - 2 mg
NDC 63868-168-20
QC
QUALITY CHOICE®
*Compare to the active ingredient in NICORETTE® Fresh MintTM
Sugar Free
Nicotine
Polacrilex
Gum USP,
2 mg (nicotine)
Stop Smoking Aid
Nicotine Polacrilex, 2 mg
FOR THOSE WHO SMOKE THEIR
FIRST CIGARETTE MORE THAN
30 MINUTES AFTER WAKING UP.
If you smoke your first cigarette
WITHIN 30 MINUTES of waking up,
use Nicotine Polacrilex Gum USP, 4 mg
2 mg
Coated
Mint Flavor
20 PIECES (2 MG EACH)
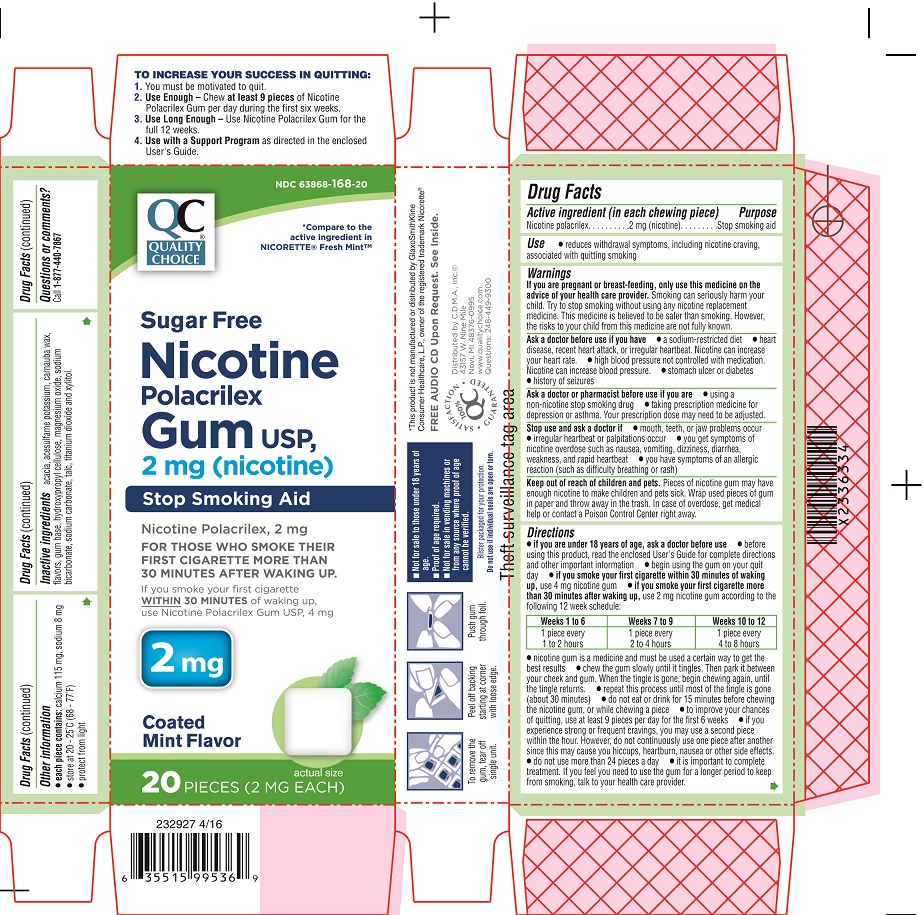
Principal Display Panel - 4 mg
NDC 63868-167-20
QC
QUALITY CHOICE®
*Compare to the active ingredient in NICORETTE® Fresh MintTM
Sugar Free
Nicotine
Polacrilex
Gum USP,
4 mg (nicotine)
Stop Smoking Aid
Nicotine Polacrilex, 4 mg
FOR THOSE WHO SMOKE THEIR
FIRST CIGARETTE WITHIN 30
MINUTES OF WAKING UP.
If you smoke your first cigarette MORE
THAN 30 MINUTES after waking up,
use Nicotine Polacrilex Gum USP, 2 mg
4 mg
Coated
Mint Flavor
20 PIECES (4 MG EACH)