WARNINGS
Lindane Shampoo should only be used in patients who cannot tolerate or have failed first-line treatment with safer medications for the treatment of lice. (See INDICATIONS AND USAGE.)
Neurologic Toxicity
Seizures and deaths have been reported following Lindane Shampoo use with repeat or prolonged application, but also in rare cases following a single application according to directions. Lindane Shampoo should be used with caution in infants, children, the elderly, and individuals with other skin conditions, and those who weigh < 110 lbs (50 kg) as they may be at risk of serious neurotoxicity.
Contraindications
Lindane Shampoo is contraindicated in premature infants and individuals with known uncontrolled seizure disorders.
Proper Use
Instruct patients on proper use of Lindane Shampoo, the amount to apply, how long to leave it on, and avoiding retreatment. Inform patients that itching occurs after the successful killing of lice and is not necessarily an indication for retreatment with Lindane Shampoo. (See DOSAGE AND ADMINISTRATION.)
DESCRIPTION
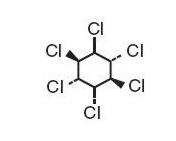
Lindane Shampoo USP, 1%, is an ectoparasiticide and ovicide effective against Pediculosis humanis capitis (head lice), Pthirus pubis (crab lice), and their ova. In addition to the active ingredient, lindane, it contains acetone, citric acid, polysorbate 60, purified water and triethanolamine lauryl sulfate to form a shampoo base. The pH may be adjusted with citric acid and/or triethanolamine. Lindane is the gamma isomer of 1,2,3,4,5,6-hexachlorocyclohexane having the following structural formula:
C6H6Cl6 M.W. 290.83
CLINICAL PHARMACOLOGY
Lindane exerts its parasiticidal action by being directly absorbed into the parasites and their ova. Feldmann and Maibach1 reported approximately 10% absorption of a lindane acetone solution applied to the forearm of human subjects and left in place for 24 hours. This vehicle was different from the approved product and the percutaneous penetration of lindane is dependent on the vehicle. Therefore, the clinical significance of these observations is unknown. Dale, et al2 reported a blood level of 290 ng/mL associated with convulsions following the accidental ingestion of a lindane-containing product. Ginsburg3 found a mean peak blood level of 28 ng/mL 6 hours after total body application of Lindane Lotion to scabietic infants and children. The half-life in blood was determined to be 18 hours.
Data available in the literature suggest that lindane has a rapid distribution phase followed by a longer β-elimination phase.1,2,3
There are no clinical dose ranging studies for Lindane Shampoo.
INDICATIONS AND USAGE
Lindane Shampoo is indicated for the treatment of head lice (infestations of Pediculosis humanis capitis), crab lice (infestations of Pthirus pubis), and their ova only in patients who
- cannot tolerate other approved therapies, or
- have failed treatment with other approved therapies.
Lindane Shampoo should be used in the context of an overall lice management program that includes:
- Visual inspection to ensure that the patient is currently infested with live lice (empty egg casings or "nits" can remain on hair shaft long after true infestation).
- Manual removal of nits using a comb designed for this purpose and/or individual removal with tweezers followed by close examination of the hair and scalp.
- Evaluation and treatment of sexual contacts simultaneously. Sexual contacts should be prescribed Lindane Shampoo only if they either have failed to respond to adequate doses of other approved therapies or are intolerant of other approved therapies.
- All recently worn clothing, underwear, pajamas, used sheets, pillowcases, and towels should be washed in very hot water or dry-cleaned.
Caregivers applying this product to patients should wear gloves less permeable to Lindane such as nitrile, latex with neoprene or sheer vinyl, and thoroughly clean hands after application. Natural latex gloves should be avoided because they are more permeable to Lindane.
Lindane Shampoo does not prevent infestation or reinfestation and should not be used to ward off a possible infestation.
CONTRAINDICATIONS
Lindane Shampoo is contraindicated for premature infants because their skin may be more permeable than that of full term infants and their liver enzymes may not be sufficiently developed to metabolize Lindane.
Lindane Shampoo is also contraindicated for patients with crusted (Norwegian) scabies and other skin conditions (e.g., atopic dermatitis, psoriasis) that may increase systemic absorption of the drug.
Lindane Shampoo is contraindicated for patients with known uncontrolled seizure disorders and for individuals with a known sensitivity to the product or any of its components.
WARNINGS
(See boxed WARNINGS.)
Seizures and deaths have been reported following Lindane Shampoo use with repeat or prolonged application, but also in rare cases following a single application according to directions.
There have been cases of adverse events reported for Lindane Shampoo and Lindane Lotion in which a serious outcome (hospitalization, disability or death) has occurred.4 In approximately 20% of these cases, the shampoo and lotion were reported to have been used according to the labeled directions. Of these cases, thirteen deaths were reported, many of which were remote from the time of actual Lindane use. Lindane toxicity, verified by autopsy was the cause of one infant's death, and was the cause of death reported for an adult in a successful suicide. The direct causes of death for the other cases were attributed to reasons other than lindane. Most of these adverse events occurred with Lindane Lotion.
Infants, children, the elderly, and individuals with other skin conditions and those who weigh < 110 lbs (50 kg) may be at a greater risk of serious neurotoxicity. (See Pediatric Use and Geriatric Use.) Animal studies have shown increased susceptibility to neurologic adverse events in younger animals. Children have a larger body surface area to volume ratio that may result in a proportionately larger systemic exposure.
Careful consideration should be given before prescribing Lindane Shampoo to patients with conditions that may increase the risk of seizure, such as HIV infection, history of head trauma or a prior seizure, CNS tumor, the presence of severe hepatic cirrhosis, excessive use of alcohol, abrupt withdrawal from alcohol or sedatives, as well as concomitant use of medications known to lower seizure threshold. (See PRECAUTIONS: Drug Interactions.)
Patients should be instructed on the proper use of Lindane Shampoo, especially the amount to apply, how long to leave shampoo on, and the need to avoid retreatment. Patients should be informed that itching may occur after the successful killing of lice and repeat treatment may not be necessary.
A Lindane Shampoo Medication Guide must be given to the patient each time Lindane Shampoo is dispensed, as required by law.
PRECAUTIONS
General
Care should be taken to avoid contact with the eyes. If such contact occurs, eyes should be immediately flushed with water. If irritation or sensitization occurs, the patient should be advised to consult a physician.
Information for Patients (and Caregivers)
- This product can be poisonous if misused.
- Other important information is found in the Medication Guide, which by law, must be dispensed with Lindane Shampoo.
- If putting Lindane Shampoo on another person, the person applying shampoo should wear less permeable gloves such as nitrile, latex with neoprene, or sheer vinyl, and thoroughly clean their hands after application. Natural latex should be avoided because it is more permeable to lindane.
- If the person applying Lindane Shampoo could be pregnant, contact with Lindane Shampoo should be avoided as much as possible.
- If the patient could be pregnant, other treatments may be preferable.
- Use Lindane Shampoo for lice only.
- The use of oil treatments, oil based hair dressings or conditioners immediately before and after applying Lindane Shampoo should be avoided. Oils can make the Lindane Shampoo go through the skin faster and possibly increase the risk of neurotoxicity (e.g., seizures).
- Information for Use
- Shake Lindane Shampoo well.
- Hair should be completely dry prior to application of Lindane Shampoo.
- Use only enough Lindane Shampoo to lightly coat the hair and scalp.
- Apply shampoo directly to dry hair without adding water. Work thoroughly into the hair and allow to remain in place for 4 minutes only. Special attention should be given to the fine hairs along the neck and behind the ears.
- After 4 minutes, add small quantities of water to hair until a good lather forms.
- Immediately rinse all lather away. Avoid unnecessary contact of lather with other body surfaces.
- Towel briskly and then remove nits with nit comb or tweezers.
- There may be some Lindane Shampoo left in the bottle. Close the bottle with the leftover Lindane Shampoo and immediately throw away the bottle in a trash can out of the reach of children.
- Do not cover the hair with anything that does not breathe, like a shower cap or towel.
- Do not ingest. Keep away from mouth and eyes. If contact with eyes occurs, immediately flush eyes with water. Do not use if open wounds, cuts or sores are present, unless specifically directed by your physician.
- Wash all recently worn clothing, underwear and pajamas, hats, and used sheets, pillowcases, and towels in very hot water or dry-clean.
- Patients may still itch after using Lindane Shampoo. This does not mean the medicine did not work. Lindane Shampoo sometimes makes this itch even worse. Other medications can be used to soothe the itch. Do not use more Lindane Shampoo.
- If there are any questions or concerns about the condition or use of the Lindane Shampoo, contact your physician.
Drug Interactions
Oils may enhance absorption of lindane, therefore, patients and caregivers applying the shampoo to others should avoid using oil treatments, or oil-based hair dressings or conditioners immediately before and after applying Lindane Shampoo.
In addition, there are many drugs that may lower the seizure threshold, and Lindane Shampoo should be prescribed with caution in patients taking these medications. Drugs that may lower the seizure threshold include, but are not limited to the following:
- Antipsychotics
- Antidepressants
- Theophylline
- Cyclosporine, mycophenolate mofetil, tacrolimus capsules
- Penicillins, imipenem, quinolone antibiotics
- Chloroquine sulfate, pyrimethamine
- Isoniazid
- Meperidine
- Radiographic contrast agents
- Centrally active anticholinesterases
- Methocarbamol
Carcinogenesis, Mutagenesis, and Fertility
Although no studies have been conducted with Lindane Shampoo, numerous long-term feeding studies have been conducted in mice and rats to evaluate the carcinogenic potential of the technical grade of hexachlorocyclohexane as well as the alpha, beta, gamma (lindane) and delta isomers. Both oral and topical applications have been evaluated. Increased incidences of neoplasms were not clearly related to administration of lindane. The results of mutagenicity tests in bacteria do not indicate that lindane is mutagenic. Lindane did not cause sister chromatid exchange in an in vivo assay. The number of spermatids in the testes of rats 2 weeks after oral administration of a single dose of 30 mg/kg body weight (12 times the estimated human exposure for scabies on a body surface area comparison and assuming 50% rat oral bioavailability and 10% human bioavailability) was significantly reduced compared to the control rats.
Pregnancy
Pregnancy Category C
All pregnancies have a risk of birth defect, loss, or other adverse event regardless of drug exposure. Predictions of fetal risk from drug exposure rely heavily on animal data. However, animal studies may fail to predict effects in humans or may overstate such risks. Even if human data are available, the data may not be sufficient to determine whether there is an increased risk to the fetus, and individual reports of adverse outcomes in pregnancy in association with a drug may not reflect a causal relationship.
Lindane Shampoo should be given to pregnant women only if clearly needed. There are no adequate and well-controlled studies of Lindane Shampoo in pregnant women. There are no known maternal or fetal health risks described if lice are not treated, but risk of transmission of the lice to other household members is an additional consideration when deciding whether to use lice treatments. Lindane is lipophilic and may accumulate in the placenta. There has been a single case report of a stillborn infant following multiple maternal exposures during pregnancy to Lindane Lotion. The relationship of the maternal exposures to the fetal outcome is unknown.
Animal data suggest that lindane may increase the likelihood of neurologic developmental abnormalities (see below), based on findings at systemic exposures close to that expected in humans when Lindane Lotion is used to treat scabies. The immature central nervous system (as in the fetus) may have increased susceptibility to the effects of the drug. Systemic exposure resulting from Lindane Shampoo applied to hair covered areas is expected to be lower than that from Lindane Lotion that covers the entire body surface area.
Data
When rats received lindane in the diet from day 6 of gestation through day 10 of lactation, reduced pup survival, decreased pup weight and decreased weight gains during lactation, increased motor activity and decreased motor activity habituation were seen in pups at 5.6 mg/kg (2 times the estimated human exposure) but not at 1.2 mg/kg. An increased number of stillborn pups was seen at 8 mg/kg, and increased pup mortality was seen at 5.6 mg/kg. No gross abnormalities were seen in this study or in a study in which rabbits received up to 20 mg/kg lindane by gavage on gestation day 6–18 (up to 10 times the human exposure on a body surface area comparison and assuming 50% rabbit oral bioavailability and 10% human bioavailability when lindane is applied to the entire body for the treatment of scabies).
Nursing Mothers
Lindane is lipophilic and is present in human breast milk, but exact quantities are not known. There may be a risk of toxicity if lindane is ingested from breast milk, or from skin absorption from mother to baby in the course of breast-feeding if Lindane Shampoo is applied topically to the chest area. Nursing mothers who require treatment with Lindane Shampoo should be advised of the potential risks and be instructed not to use the product on the skin as would be done for treatment of scabies. They should also be counseled to interrupt breast-feeding, with expression and discarding of milk, for at least 24 hours following use.
Pediatric Use
Animal data demonstrated increased risk of adverse events in the young across species. Pediatric patients have a higher surface to volume ratio and may be at risk of greater systemic exposure when Lindane Shampoo is applied. Infants and children may be at an even higher risk due to immaturity of organ systems such as skin and liver. Lindane Shampoo should be used with caution in patients who weigh less than approximately 110 lbs (50 kg) and especially in infants. Lindane Shampoo is indicated only for the treatment of lice; patients with scabies should use Lindane Lotion according to the labeled instructions.
Geriatric Use
There have been no studies of Lindane Shampoo in the elderly. There are four postmarketing reports of deaths in elderly patients treated with Lindane Lotion for the indication of scabies. Two patients died within 24 hours of Lindane Lotion application, and the third patient died 41 days after application of Lindane Lotion, having suffered a seizure on the day of death. A fourth patient died of an unreported cause of death on the same day that Lindane Lotion treatment for scabies was administered.
ADVERSE REACTIONS
Central nervous system stimulation ranging from dizziness to seizures, has been reported particularly with use of Lindane Lotion. Although seizures were almost always associated with ingestion or misuse of the product (to include repeat treatment), seizures and deaths have been reported when Lindane Shampoo was used according to directions. Irritant dermatitis from contact with this product has also been reported. (See WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION.)
OVERDOSAGE
Contact the closest Poison Control Center in the event of suspected overdosage with Lindane Shampoo.
If accidental ingestion occurs, prompt gastric lavage should be instituted. However, since oils enhance absorption, saline cathartics for intestinal evacuation should be given rather than oil laxatives. If central nervous system manifestations occur, they may be antagonized by the administration of pentobarbital, phenobarbital, or diazepam.
DOSAGE AND ADMINISTRATION
Most patients will require only 1 ounce of Lindane Shampoo. Based on the length and density of hair, some patients may require 2 ounces of Lindane Shampoo.
Apply shampoo directly to dry hair without adding water. Work thoroughly into the hair and allow to remain in place for 4 minutes only. Special attention should be given to the fine hairs along the neck. After 4 minutes, add small quantities of water to hair until a good lather forms. Immediately rinse all lather away. Avoid unnecessary contact of lather with other body surfaces. Do not prescribe more than 2 ounces for larger adults. Do not retreat. (See boxed WARNINGS.)
Patients should be provided specific information on use of product. (See PRECAUTIONS: Information for Patients and Lindane Shampoo Medication Guide.)
A Lindane Shampoo Medication Guide must be given to the patient each time LINDANE Shampoo is dispensed as required by law. The Lindane Shampoo Medication Guide is an important part of the risk management program for the patient.
HOW SUPPLIED
Lindane Shampoo USP, 1% is supplied in 2 fl oz (60 mL) NDC 61748-400-02 bottles.
SHAKE WELL BEFORE USING
REFERENCES
- Feldmann, R.J. and Maibach, H.I., Toxicol. Applied. Pharmacol., 28:126, 1974.
- Dale, W.E., Curly, A. and Cueto, C. Life Sci 5:47, 1966.
- Ginsburg, C.M., et al., J. Pediatr. 91:6, 998–1000, 1977.
- FDA AERS database search, January 2003
Manufactured by:
AL&S, LLC
De Kalb, MS 39328
Marketed by:
VersaPharm Incorporated
Marietta, GA 30062
IVP40002-00
Issued: 05-24-2011
PHARMACIST—PATIENT MEDICATION GUIDE PROVIDED BELOW
MEDICATION GUIDE
Lindane (LIHN-dane) Shampoo USP, 1%
You must read and follow all instructions before using Lindane Shampoo. Read the information you get every time you or a family member get Lindane Shampoo. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. If you have any questions about Lindane Shampoo, ask your doctor or pharmacist.
What is the most important information I should know about Lindane Shampoo?
Lindane Shampoo is a poison if you do not use it the right way. Lindane Shampoo goes through your skin and can affect your brain and nerves. Lindane Shampoo can cause seizures, also called convulsions, "fits" or epilepsy.
- Seizures and death can happen in people who use Lindane Shampoo too much or too often.
- Seizures can happen in some people even if they use Lindane Shampoo exactly as directed.
If you or a family member has a seizure while using Lindane Shampoo, get emergency help right away.
- Do not use Lindane Shampoo unless
- You have lice and another medicine did not work for you, or
- You cannot use other, safer medicines to treat your lice
- Do not use Lindane Shampoo more than 1 time to treat an attack of lice. Do not use Lindane Shampoo to treat a second attack that comes soon after the first episode. No one knows a safe time to reuse Lindane Shampoo. Even if you still itch even after using Lindane Shampoo, do not use more or use it again. Lice (bugs) can make you itch for some time even after all of the bugs are dead.
- Do not use more Lindane Shampoo than your doctor tells you.
- Do not keep Lindane Shampoo on your hair for more than 4 minutes.
- Do not put Lindane Shampoo in your mouth because it is a poison if taken by mouth. If you get Lindane Shampoo in your mouth or swallow Lindane Shampoo, call your area Poison Control Center right away and get emergency help.
What is Lindane Shampoo?
Lindane Shampoo is a medicine that is used to treat lice. It kills lice and their eggs. Lice are very small bugs that attach to the skin on your head or pubic (crotch) area and lay eggs called nits in your hair. Some people call crotch lice "crabs". Lice can cause severe itching. Lindane Shampoo gets into the lice and the nits and kills them. It also goes through your skin. Lindane Shampoo is used only after safer medicines have not made your lice go away. The only time Lindane Shampoo is used first is when someone cannot use safer medicines, which may include permethrin and crotamiton.
Lindane Shampoo is mainly for adults and children who weigh at least 110 pounds. If you weigh less than 110 pounds use Lindane Shampoo only if your doctor thinks it is really needed. People who weigh less than 110 pounds and the elderly have higher chances for side effects because more Lindane may go through their skin.
Who should not use Lindane Shampoo?
Do not use Lindane Shampoo:
- if you do not have lice. Lindane Shampoo does not stop you from getting lice. Lindane Shampoo only kills the lice you already have.
- if you have or have ever had seizures, also called convulsions, "fits" or epilepsy, especially if they have been hard to control.
- if you have used Lindane Shampoo in the past few months. You should see your doctor if you think that you need another treatment.
- unless it is the only medicine you can use for lice.
- if you had a bad reaction to Lindane Shampoo before. Do not use Lindane Shampoo again.
- if you have open sores or crusted (scabby) sores on the skin around your head and neck, or lots of broken skin.
- if you need to treat a premature or young baby. More of the applied Lindane can go through the skin of babies and go to their brains where it can harm them.
- if you have scabies. These need a different medicine that you use in a different way.
- if you are allergic to Lindane Shampoo or any of its ingredients. The active ingredient is lindane. See the end of this Medication Guide for a list of all the ingredients in Lindane Shampoo.
- while you are breast-feeding. Lindane Shampoo can get in your milk and may be fed to your baby. Your baby may get sick. Ask your doctor for a safer medicine. If you use Lindane Shampoo, pump your breast milk and throw the milk away for at least 24 hours after using the medicine. During this time, feed your baby formula or breast milk that you stored from before you used Lindane Shampoo.
Tell your doctor if you:
- used Lindane Shampoo in the past few months.
- ever had a seizure or problem that could increase your chances of getting a seizure (like a head injury, tumor in your brain or spinal cord, cirrhosis of the liver, or heavy alcohol drinking.)
- have HIV or AIDS. Lindane Shampoo may cause seizures even if you never had them before.
- are pregnant. Lindane Shampoo can reach your baby and may harm it. Ask your doctor for a safer medicine. Use Lindane Shampoo only if needed.
- have a sexual partner and your lice (crabs) are in your pubic (crotch) area. Your partner should get checked and treated for lice so they don't give them back to you. Don't share your Lindane Shampoo with your partner.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may increase your chances of having a seizure if you take them while using Lindane Shampoo. Especially tell your doctor if you take medicines called sedatives (drugs to help you sleep).
How do I use Lindane Shampoo?
Before you put it on:
- Make sure you know how to use it exactly as your doctor prescribes.
- If you are putting Lindane Shampoo on another person, wear special gloves made of nitrile, latex with neoprene, or sheer vinyl. Do not use natural latex gloves because more Lindane can go through that kind of glove. Keep the gloves on until the Lindane Shampoo is washed out of your hair. Wash your hands well when you are done.
- Make sure your hair and skin on your head and neck do not have any other shampoo, cream, or oil on it. Oils can make the Lindane Shampoo go through your skin faster and may increase the risk of seizures.
When you put it on:
- Shake the bottle of Lindane Shampoo well.
- Make sure your hair is clean and dry before using Lindane Shampoo, but do not wash your hair within 1 hour before using Lindane Shampoo. Use regular shampoo without conditioner and dry your hair.
- Use just enough Lindane Shampoo on your dry hair to wet your hair and scalp. Do not add water to your hair at this time. Also, put Lindane Shampoo on the short hairs at the back of your neck.
- Keep Lindane Shampoo on your hair for 4 minutes. Use a watch or clock to time yourself.
- Do not wear a shower cap or any covering on your head while you wait for the 4 minutes to pass.
- Close the bottle with the leftover Lindane Shampoo and throw it away in a trash can out of the reach of children.
When you are supposed to wash it off:
- After 4 minutes has passed, soap up or lather the Lindane Shampoo. Use a small amount of warm water to do this. Hot water is not safe. Then wash the Lindane Shampoo off your head. Again, use warm, but not hot water. Do not leave any Lindane Shampoo on your head or hair. It will not kill more of the lice and may continue to go through your skin and cause serious problems, such as seizures.
After you wash off Lindane Shampoo:
- Dry your hair with a towel. Use a special comb called a nit comb or tweezers to remove the dead nits (lice eggs) from your hair. Someone else will probably have to do this for you.
- All recently worn clothing, underwear, pajamas, hats, used sheets, pillowcases, and towels should be washed in very hot water or dry-cleaned.
- Do not use Lindane Shampoo again. If you think you need to use it again, you must check with your doctor to find out if and when it is most safe.
You may still itch after you have used Lindane Shampoo. This does not mean you need more Lindane Shampoo. Even after all the lice (bugs) are dead, they can still make your skin itch for a long time. Lindane Shampoo sometimes makes this itch even worse. Talk to your doctor about things you can do to soothe the itch.
What should I avoid while using Lindane Shampoo?
- Do not get Lindane Shampoo in your eyes. If you do, rinse your eyes with water right away. Get medical help if your eyes keep hurting.
- Do not get Lindane Shampoo on your hands. Wear special gloves made of nitrile, latex with neoprene, or sheer vinyl. Do not use natural latex gloves. Wash your hands well when you are done.
- If you are pregnant, do not use Lindane Shampoo unless you have talked to your doctor about using it. Avoid putting Lindane Shampoo on others if you are pregnant. See the special glove advice above if you have to put Lindane Shampoo on others.
- Do not use oils on your skin or hair, just before or after using Lindane Shampoo. Oils include oil-based hair products and conditioners.
What are the possible side effects of Lindane Shampoo?
Lindane Shampoo may cause serious side effects such as seizures (convulsions, fits) or death (See the section, "What is the most important information I should know about Lindane Shampoo?"). Lindane Shampoo can also make you feel sleepy, dizzy, or can cause body shaking that you cannot control.
The most common side effects of Lindane Shampoo are:
- Itching skin
- Burning skin
- Dry skin
- A skin rash
These are not all of the possible side effects of Lindane Shampoo. For more information, ask your doctor or pharmacist.
General Information about Lindane Shampoo:
Medicines are sometimes prescribed for purposes other than those listed in Medication Guides. Do not use Lindane Shampoo for any condition for which it was not prescribed. Do not give Lindane Shampoo to other people, even if they have the same symptoms that you have. It may harm them. Keep Lindane Shampoo and all medicines out of the reach of children.
This Medication Guide summarizes the most important information about Lindane Shampoo. If you want more information, talk with your doctor. You can ask your doctor or pharmacist for information about Lindane Shampoo that is written for health professionals.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1800-FDA-1088.
What are the ingredients in Lindane Shampoo?
Active Ingredient: Lindane.
Inactive Ingredients: acetone, citric acid, polysorbate 60, purified water and triethanolamine lauryl sulfate.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Lindane is only available by a prescription from your doctor.
Manufactured by:
AL&S, LLC
De Kalb, MS 39328
Marketed by:
VersaPharm Incorporated
Marietta, GA 30062
GVP40002-00
Issued: 05-24-2011
