PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
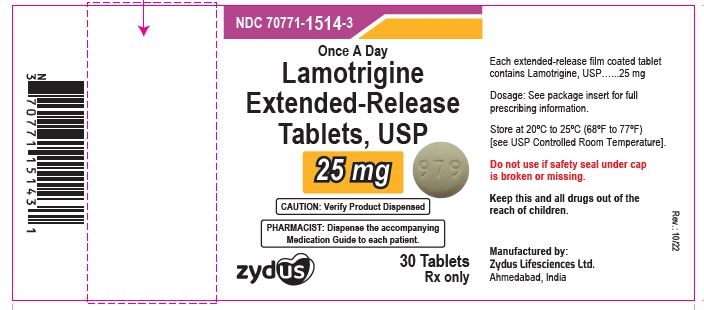
Lamotrigine extended-release tablets, 25 mg
Rx only
30 tablets
Lamotrigine extended-release tablets, 50 mg
Rx only
30 tablets

Lamotrigine extended-release tablets, 100 mg
Rx only
30 tablets

Lamotrigine extended-release tablets, 200 mg
Rx only
30 tablets

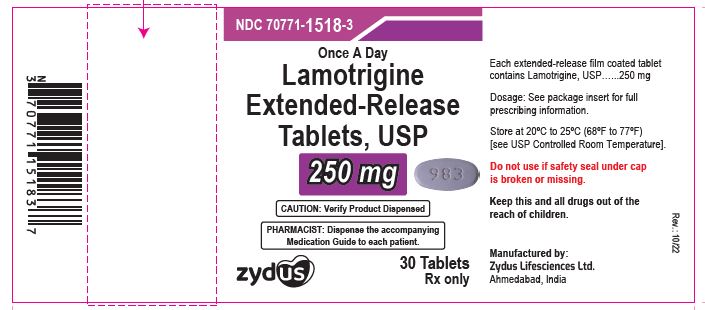
Lamotrigine extended-release tablets, 250 mg
Rx only
30 tablets
Lamotrigine extended-release tablets, 300 mg
Rx only
30 tablets
