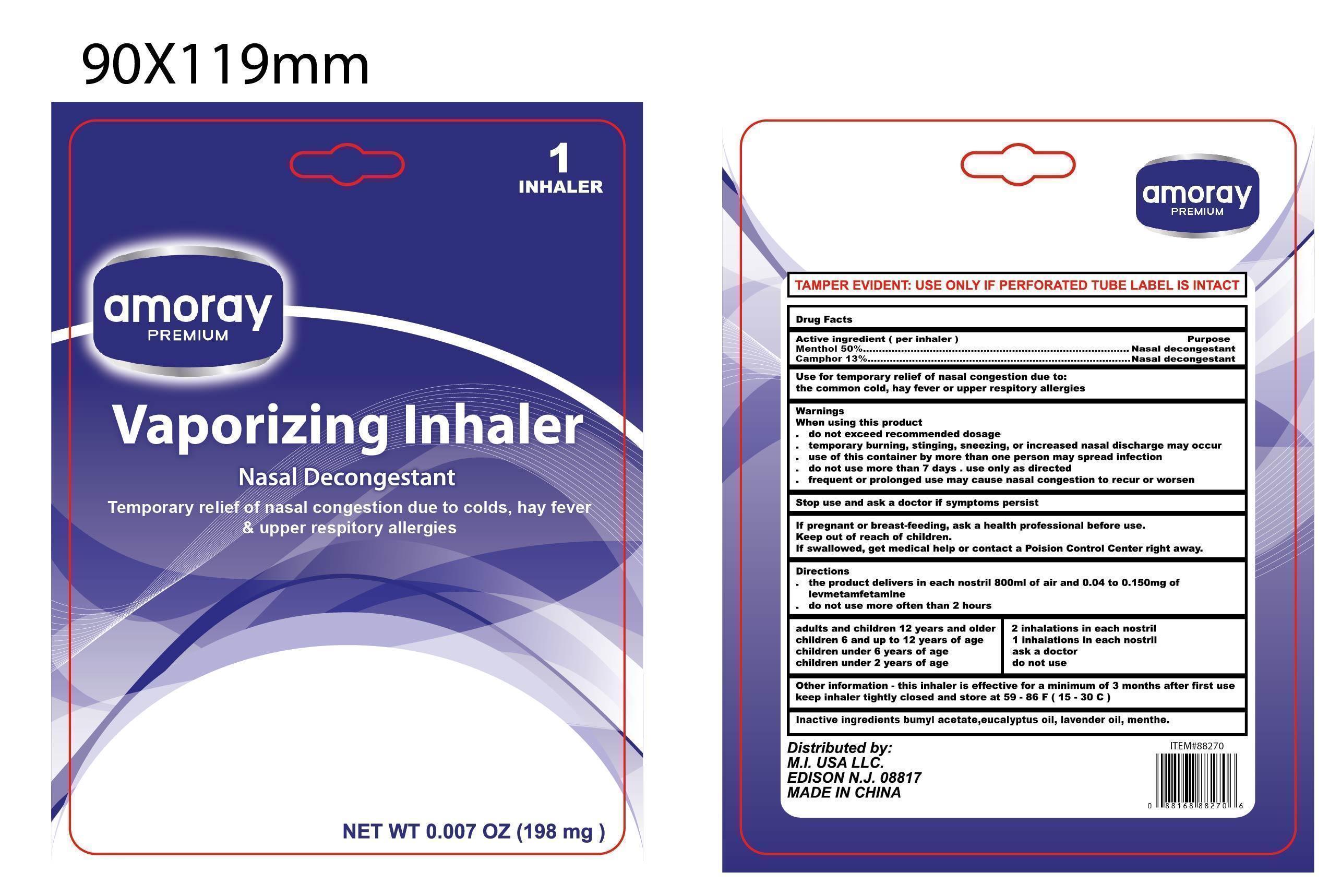
Active Ingredients
Menthol 50% ................... Nasal Decongestant
camphor 13% .................. Nasal Decongestant
INDICATIONS:
USE FOR TEMPORARY RELIEF OF NASAL CONGESTION DUE TO:
The COMMON COLD, HAY FEVER OR UPPER RESPIRATORY ALLERGIES.
Warnings:
When using this product
- DO NOT EXCEED RECOMMENDED DOSAGE.
- Temporary BURNING, STINGING, SNEEZING, OR AN INCREASED IN NASAL DISCHARGE may occur.
- THE USE OF THIS CONTAINER BY MORE THAN ONE PERSON MAY SPREAD INFECTION.
- DO NOT USE THIS PRODUCT FOR MORE THAN 7 DAY. USE ONLY AS DISRECTED.
- FREQUENT OR PROLONGED USE MAY CAUSE NASAL CONGESTION TO RECUR OR WORSEN.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. if swallowed, get medical help or contact a Poision Control Center right away.
DIRECTIONS:
- THE PRODUCT DELIVERS IN EACH 800ML OF AIR 0.04 TO 0.150 MG OF LEVMETAMFETAMINE.
- Do not use more often than 2 hours.
ADULTS AND CHILDREN 12 YEARS OF AGE AND OVER: 2 INHALATIONS IN EACH NOSTRIL.
CHILDREN 6 TO 12 YEARS OF AGE (WITH ADULT SUPERVISION): 1 INHALATION IN EACH NOSTRIL.
CHILDREN UNDER 6 YEARS OF AGE: CONSULT A DOCTOR.
Childre nuder 2 years of age: DO NOT USE.