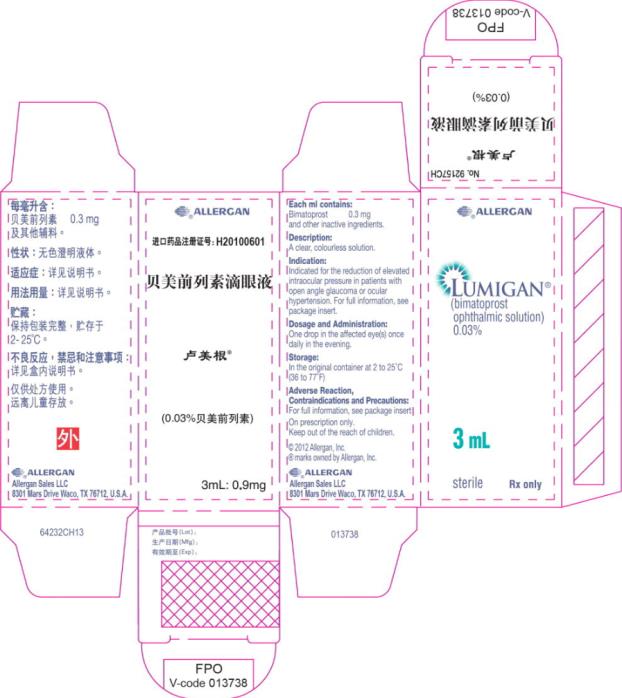
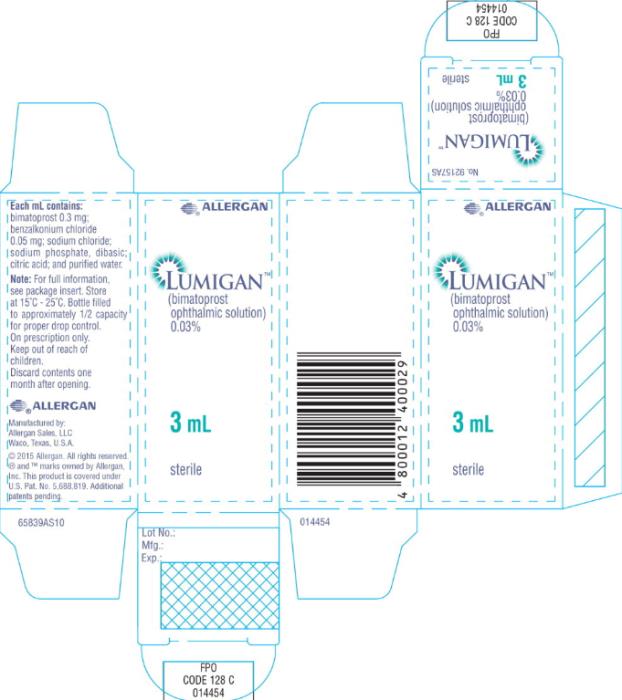
Principal Display Panel - 0.3 mg/1 mL Carton Label
®ALLERGAN
LUMIGAN®
(bimatoprost
ophthalmic solution)
0.03%
3 mL
sterile Rx only
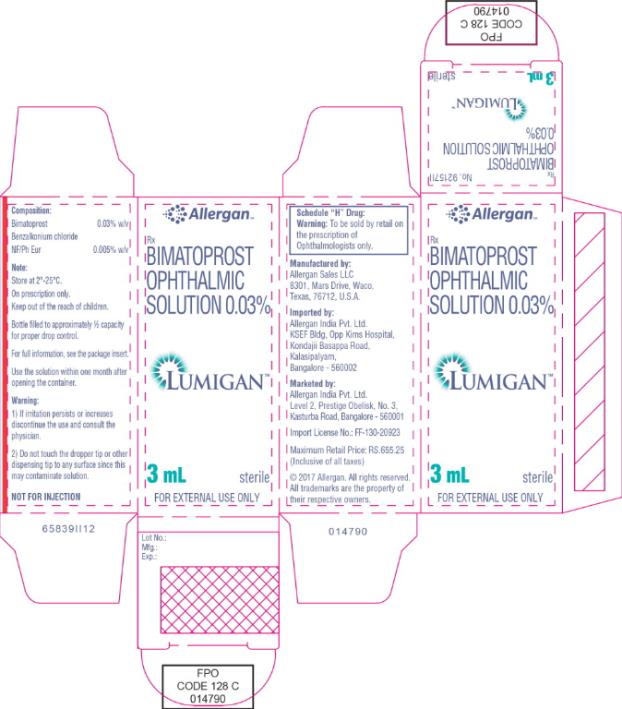
Principal Display Panel - 0.3 mg/1 mL Carton Label
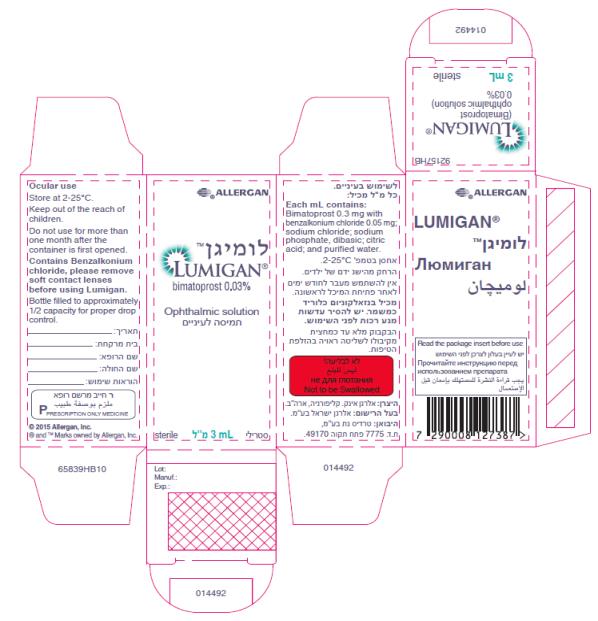
ALLERGAN™
Rx
bimatoprost
ophthalmic solution
0.03%
LUMIGAN™
3 mL
sterile
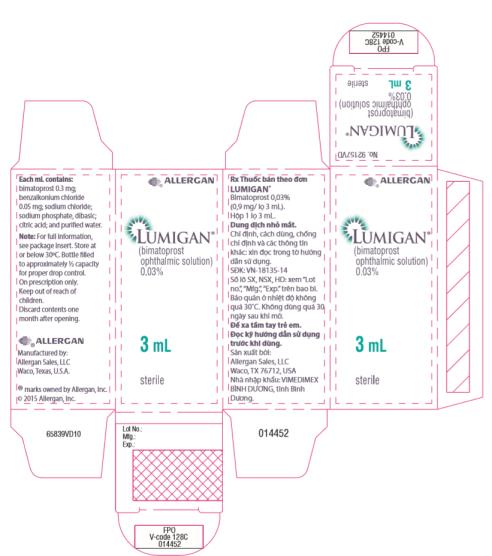
Principal Display Panel - 0.3 mg/1 mL Carton Label
®ALLERGAN
LUMIGAN™
Rx
(BIMATOPROST
OPHTHALMIC
SOLUTION)
0.03%
3 mL
sterile
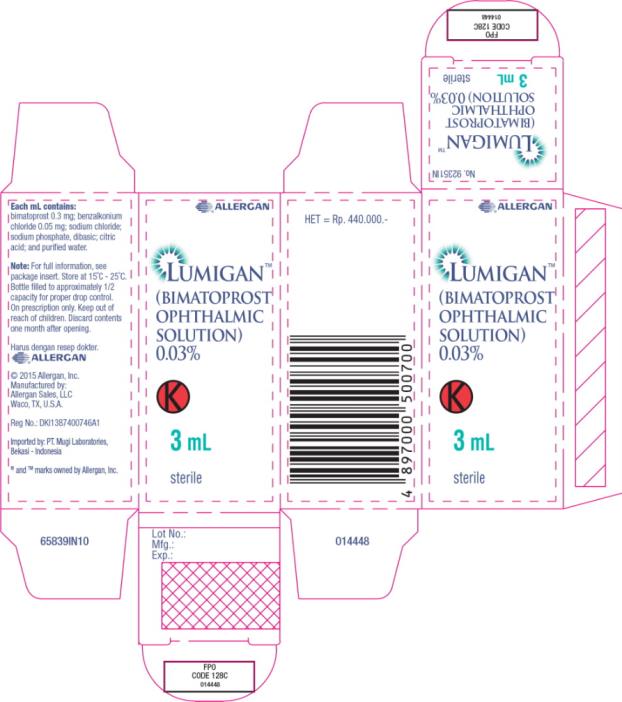
Principal Display Panel - 0.3 mg/1 mL Carton Label
®ALLERGAN
LUMIGAN™
(BIMATOPROST
OPHTHALMIC
SOLUTION)
0.03%
K
3 mL
sterile