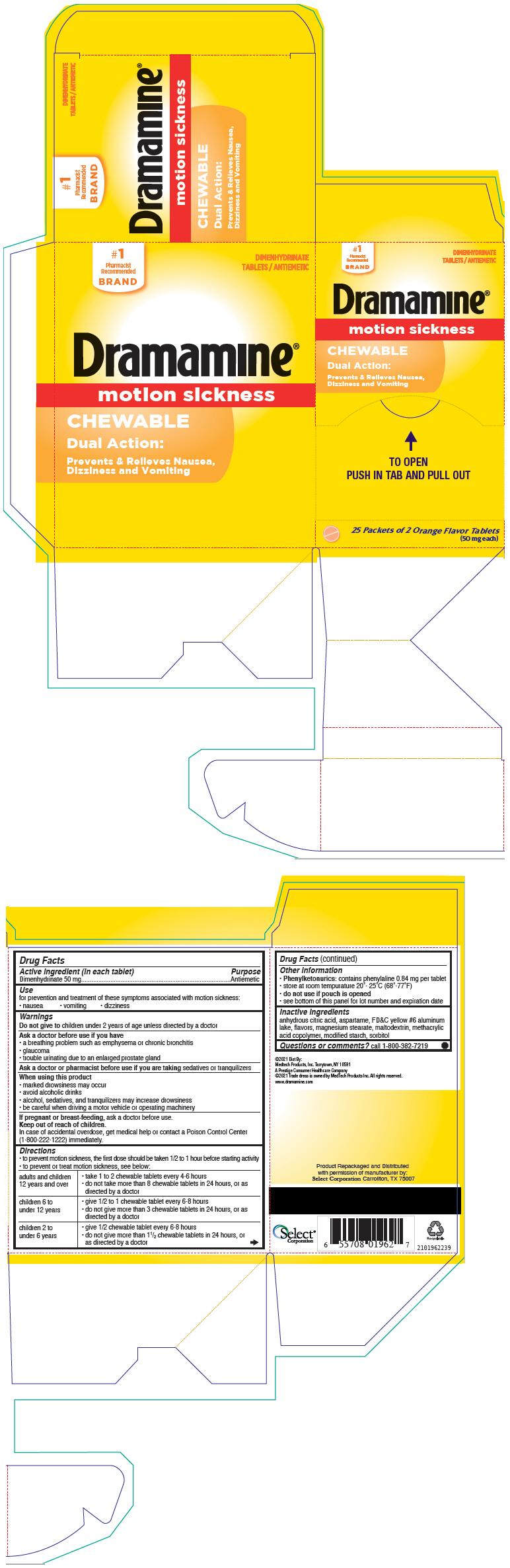
Use
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Do not give to children under 2 years of age unless directed by a doctor
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- to prevent motion sickness, the first dose should be taken 1/2 to 1 hour before starting activity
- to prevent or treat motion sickness, see below:
| adults and children 12 years and over |
|
| children 6 to under 12 years |
|
| children 2 to under 6 years |
|
Other information
- Phenylketonurics: contains phenylaline 0.84 mg per tablet
- store at room tempurature 20°- 25°C (68°-77°F)
- do not use if pouch is opened
- see bottom of this panel for lot number and expiration date
Inactive ingredients
anhydrous citric acid, aspartame, FD&C yellow #6 aluminum lake, flavors, magnesium stearate, maltodextrin, methacrylic acid copolymer, modified starch, sorbitol
Dist By:
Medtech Products, Inc. Tarrytown, NY 10591
Product Repackaged and Distributed
with permission of manufacturer by:
Select Corporation Carrollton, TX 75007
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Packet Carton
#1
Pharmacist
Recommended
BRAND
DIMENHYDRINATE
TABLETS / ANTIEMETIC
Dramamine®
motion sickness
CHEWABLE
Dual Action:
Prevents & Relieves Nausea,
Dizziness and Vomiting
TO OPEN
PUSH IN TAB AND PULL OUT
25 Packets of 2 Orange Flavor Tablets
(50 mg each)