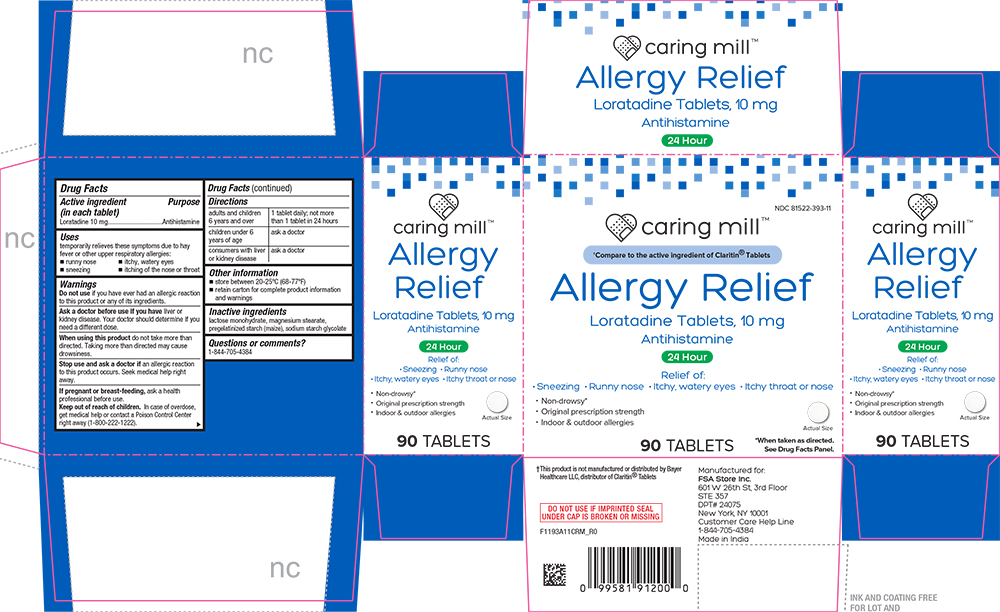
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- store between 20 to 25°C (68 to 77°F)
- retain carton for complete product information and warnings
Inactive ingredients
lactose monohydrate, magnesium stearate, pregelatinized starch (maize), sodium starch glycolate
PRINCIPAL DISPLAY PANEL
NDC 81522-393-11
caring mill™
†Compare to the active ingeedient of Claritin® Tablets
Allergy Relief
Loratadine Tablets, 10 mg
Antihistamine
25 Hour
Relief of:
• Sneezing • Runny nose • Itchy, watery eyes • Itchy throat or nose
• Non-drowsy*
• Original prescription strength
• Indoor & outdoor allergies
90 TABLETS
Actual Size
*When taken as directed. See Drug Facts Panel