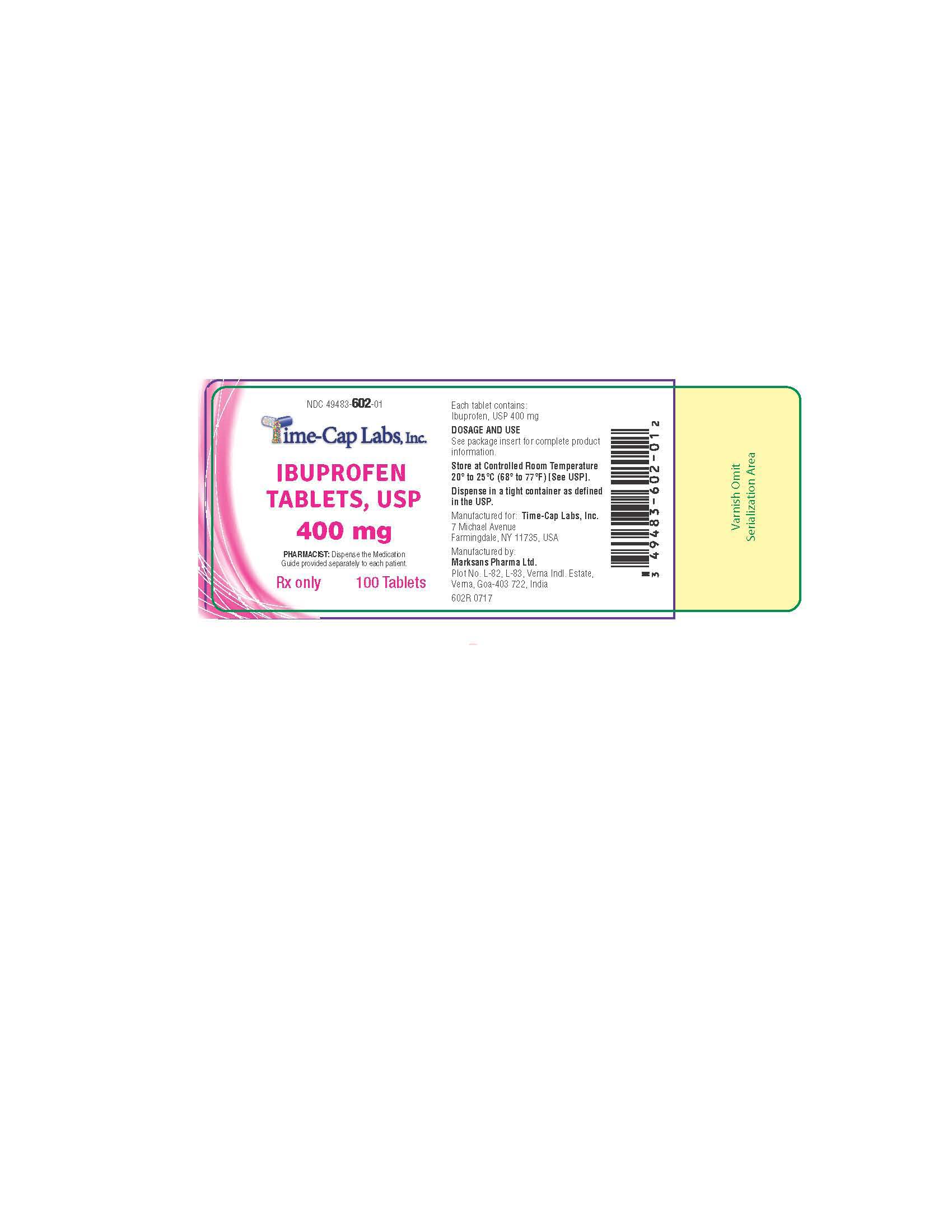
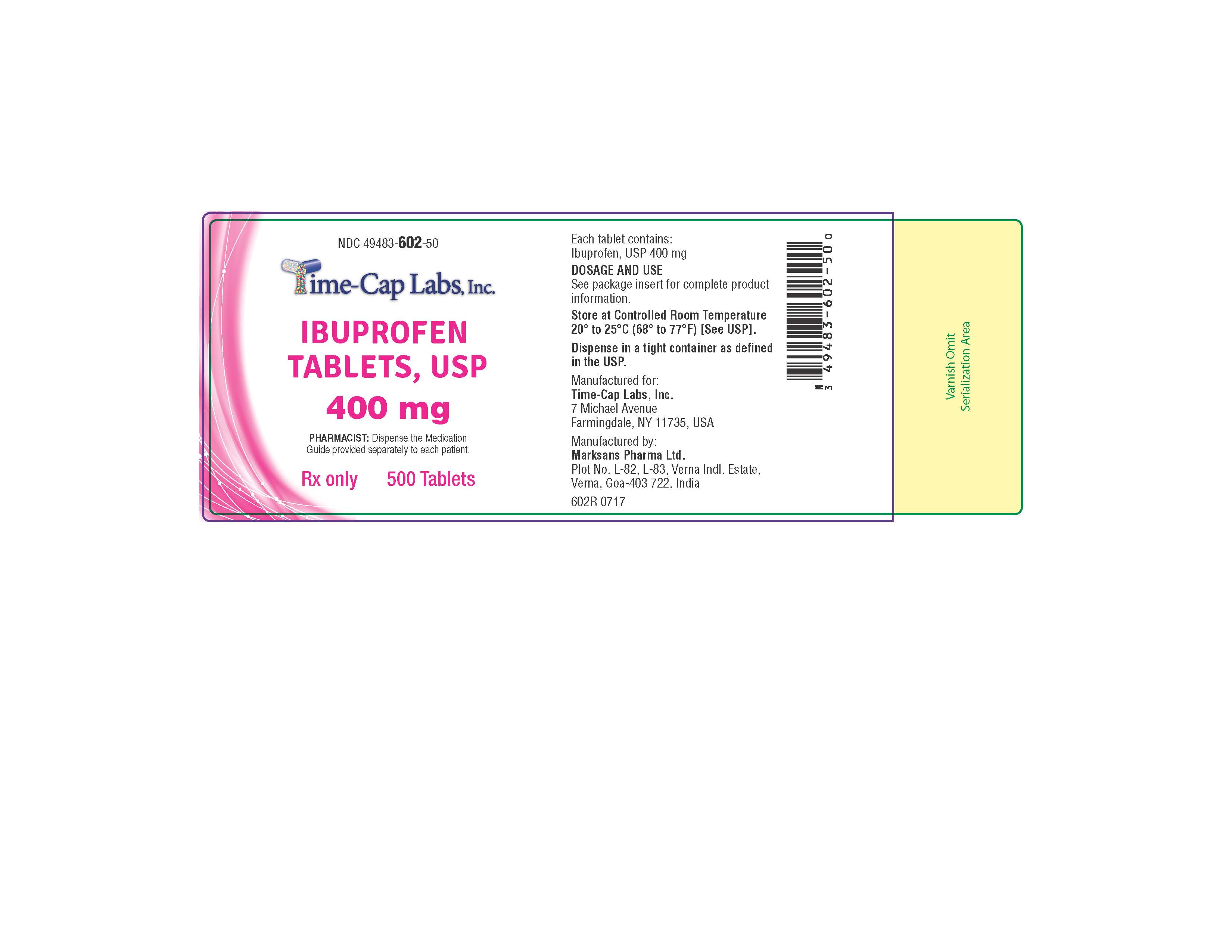
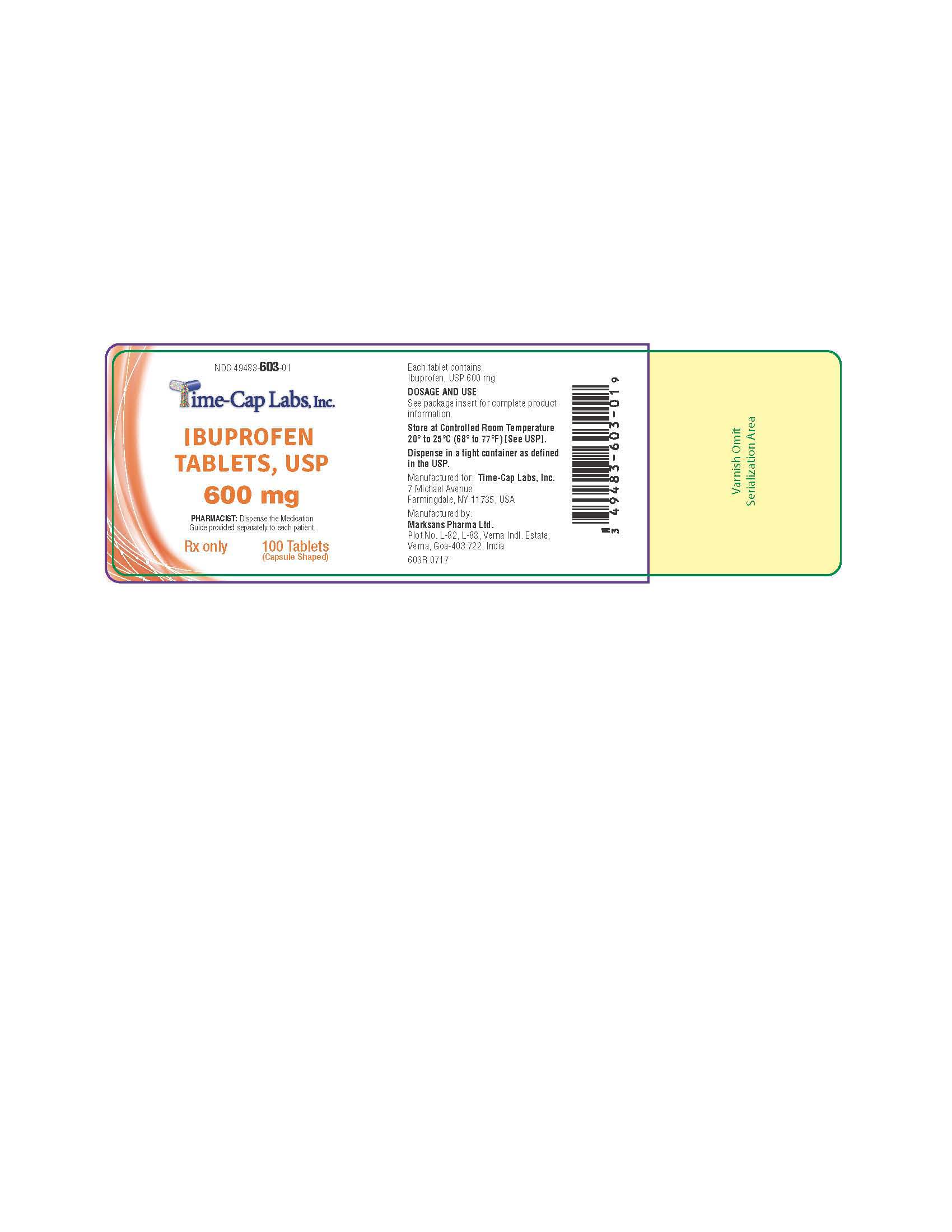
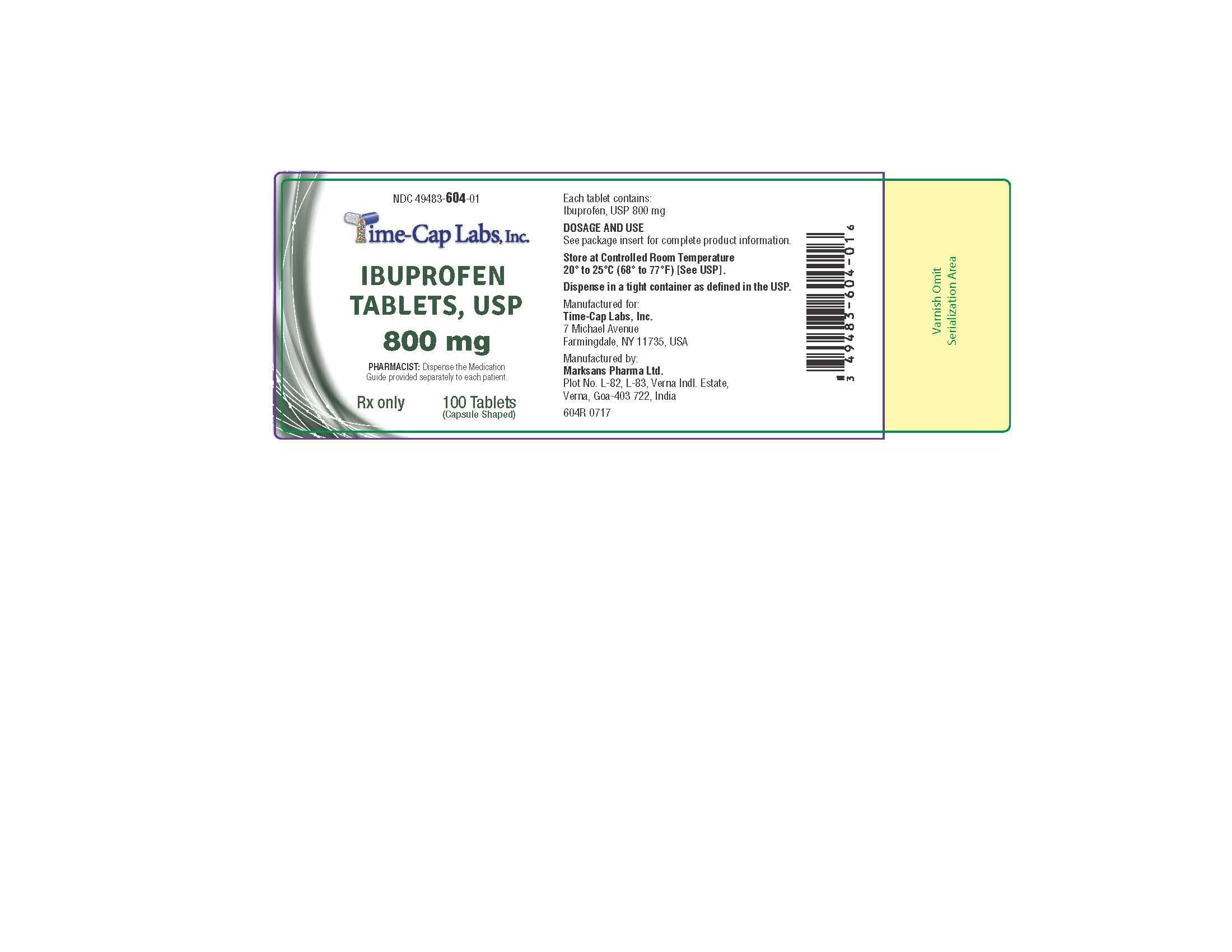
HOW SUPPLIED
400mg (white to of white, round, biconvex, film coated tablets debossed with '121' on one side and plain on the other side) Bottles of 100 & 500
HOW SUPPLIED
600mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with '122' on one side and plain on the other side) Bottles of 30, 50, 100 & 500