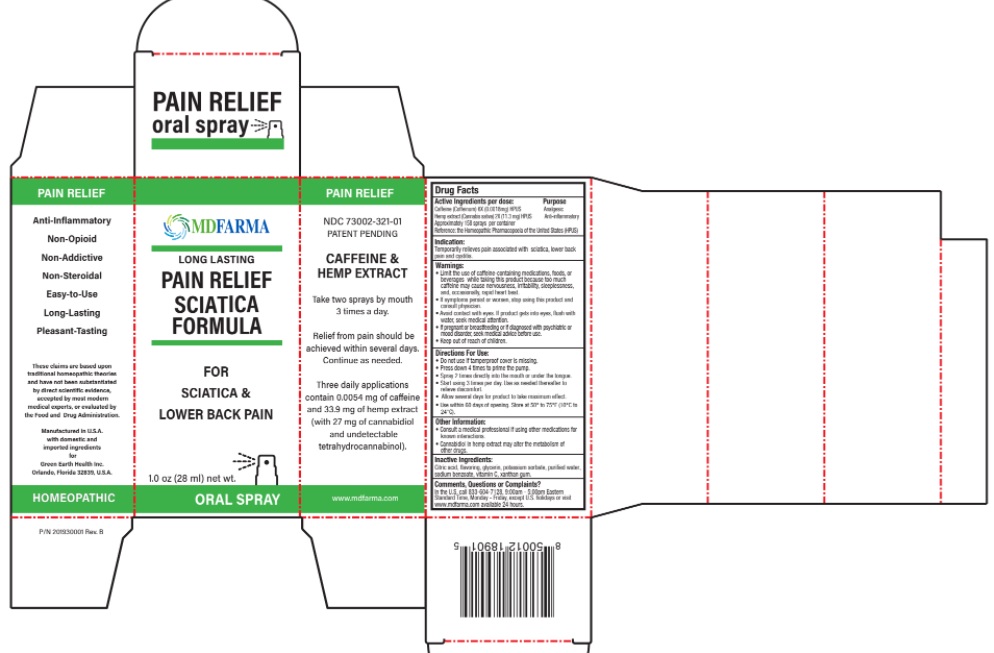
Active Ingredients Purpose
Caffeine (Coffeinum) 6X (0.0018mg) HPUS Analgesic
Hemp extract (Cannabis sativa) 2X (11.3 mg) HPUS Anti-inflammatory
Approximately 158 sprays per container
Reference: the Homeopathic Pharmacopoeia of the United States (HPUS)
Warnings:
• Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
• If symptoms persist or worsen, stop using this product and consult physician.
• Avoid contact with eyes. If product gets into eyes, flush with water, seek medical attention.
• If pregnant or breastfeeding or if diagnosed with psychiatric or mood disorder, seek medical advice before use.
• Keep out of reach of children.
Directions For Use:
- Do not use if tamperproof cover is missing
- Press down 4 times to prime the pump.
- Spray 2 times directly into the mouth or under the tongue.
- Start using 3 times per day. Use as needed thereafter to relieve discomfort.
- Allow several days for product to take maximum effect.
- Use within 60 days from opening. Store at 50° to 75°F (10°C to 24°C)
Other Information:
- Consult a medical professional if using other medications for known interactions.
- Cannabidiol in hemp extract may alter the metabolism of other drugs.