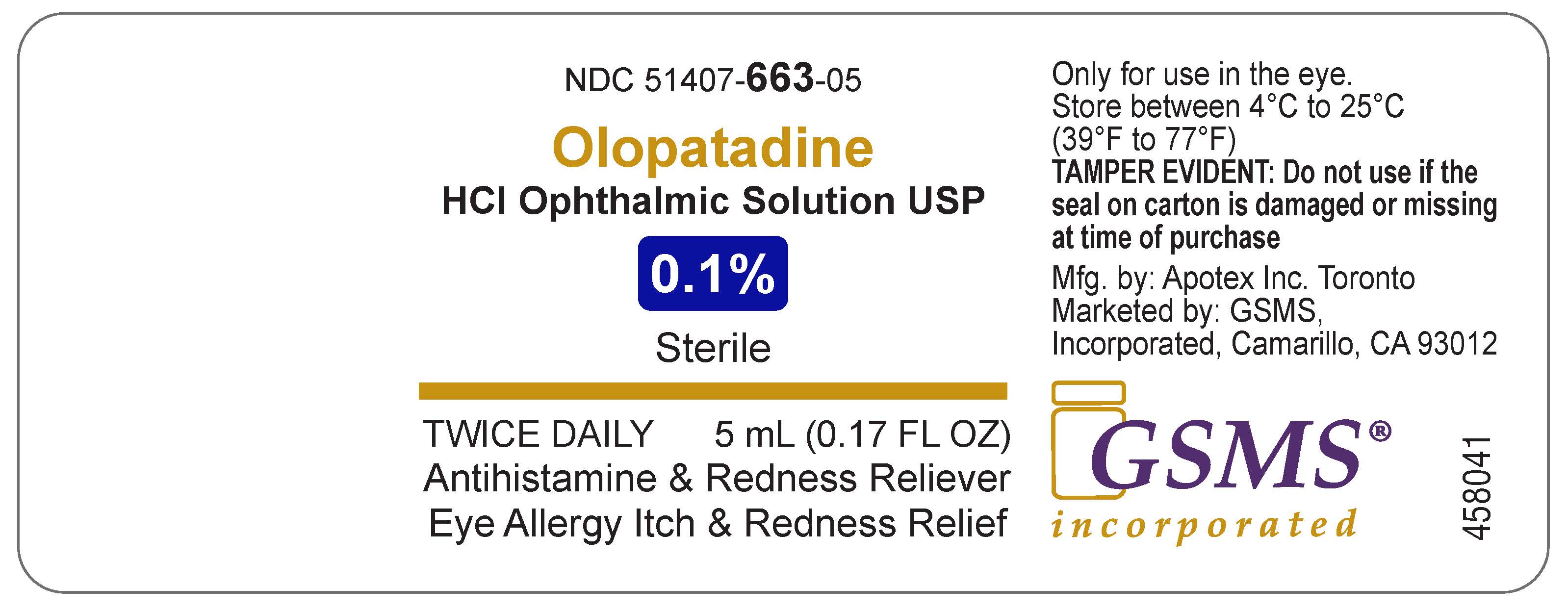
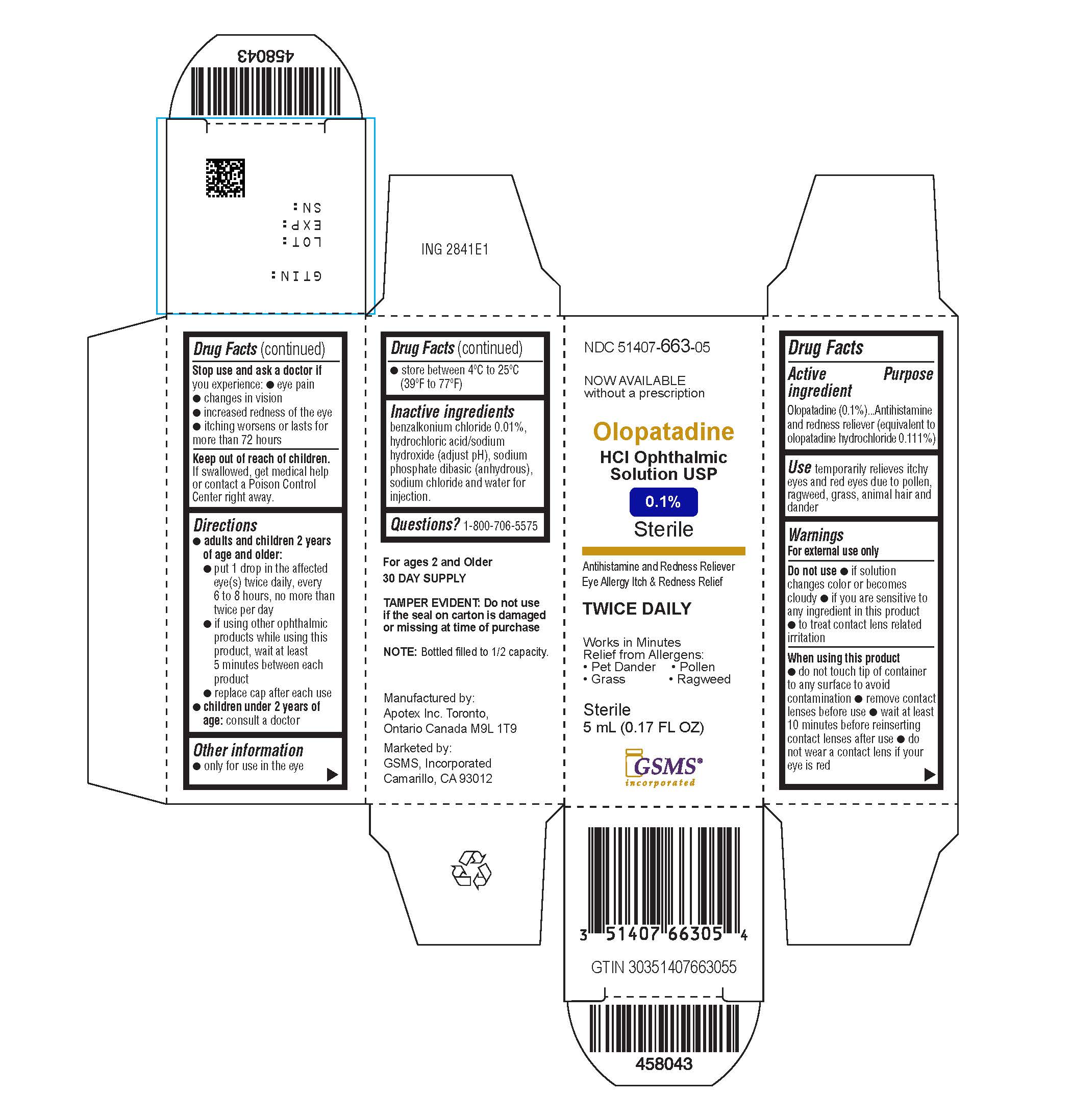
Active ingredient(in each tablet)
Olopatadine (01.%) (equivalent to olopatadine hydrochloride 0.111%)
Purpose
Antihistamine and redness reliever
Uses
Temporarily relieves itchy and red eyes due to pollen, ragweed, grass, animal hair and dander.
Warnings
For external use only
Do not use
- If solution changes color or becomes cloudy
- If you are sensitive to any ingredient in this product
- To treat contact lens related irritation
When using this product
- Do not touch tip of container to any surface to avoid contamination
- Remove contact lenses before use
- Wait at least 10 minutes before reinserting contact lenses after use
- Do not wear a contact lens if your eye is red
Stop use and ask a doctor ifyou experience
:
- Eye pain
- Changes in vision
-
Increased redness of the eye
- Itching worsens or lasts for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
adults and children 2 years and older:
- put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
-
children under 2 years of age:
Consult a doctor
Other information
- Only for use in the eye
- Store between 4ºC to 25ºC (39ºF to 77ºF)
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate (anhydrous), hydrochloric acid and/or sodium hydroxide (to adjust pH), purified water and sodium chloride
Questions?
1-800-706-5575
Principal Display Panel
TWICE DAILY RELIEF
Olopatadine HCl Ophthalmic Solution, USP 0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
NDC 51407-663-05
Golden State Medical Supply, Inc.

