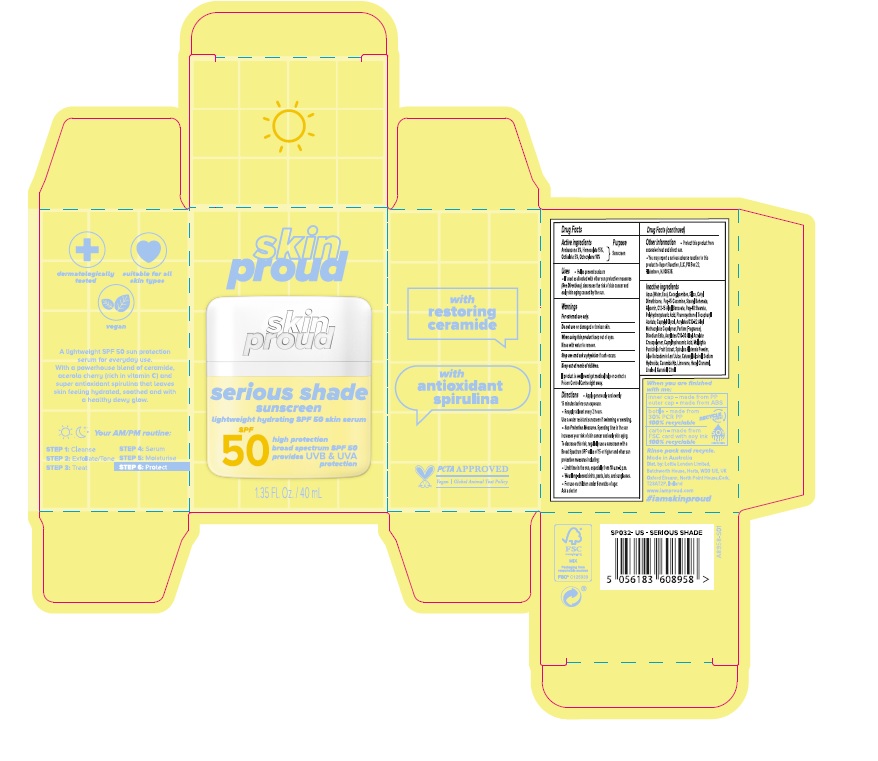
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures ( See Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply generously and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- For use on children under 6 months of age: Ask a doctor
Other information
- Protect this product from excessive heat and direct sun.
- You may report a serious adverse reaction to this product to Report Reaction, LLC, PO Box 22, Plainsboro, NJ 08536.
Inactive ingredients
Aqua (Water, Eau), Cocoglycerides, Silica, Cetyl Dimethicone, Peg-15 Cocamine, Stearyl Behenate, Glycerin, C12-15 Alkyl Benzoate, Peg-40 Stearate, Polyhydroxystearic Acid, Phenoxyethanol, Tocopheryl Acetate, Caprylyl Glycol, Acrylates/C12-22 Alkyl Methacrylate Copolymer, Parfum (Fragrance), Disodium Edta, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylhydroxamic Acid, Malpighia Punicifolia Fruit Extract, Spirulina Platensis Powder, Aloe Barbadensis Leaf Juice, Cetearyl Alcohol, Sodium Hydroxide, Ceramide Np, Limonene, Hexyl Cinnamal, Linalool, Geraniol, Citral.