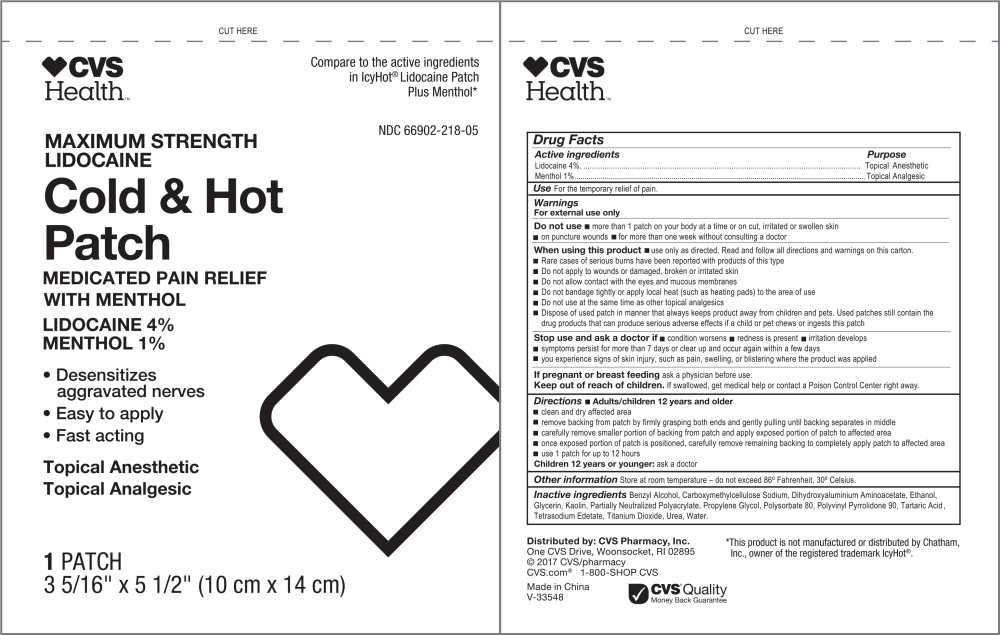
Warnings
For external use only
Do not use
- more than 1 patch on your body at a time or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- Rare cases of serious burns have been reported with products of this type
- Do not apply to wounds or damaged, broken or irritated skin
- Do not allow contact with the eyes and mucous membranes
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use
- Do not use at the same time as other topical analgesics
- Dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug products that can produce serious adverse effects if a child or pet chews or ingests this patch
Directions
- Adults/children 12 years and older
- clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- use 1 patch for up to 12 hours
Inactive ingredients
Benzyl Alcohol, Carboxymethylcellulose Sodium, Dihydroxyaluminium Aminoacetate, Ethanol, Glycerin, Kaolin, Partially Neutralized Polyacrylate, Propylene Glycol, Polysorbate 80, Polyvinyl Pyrrolidone 90, Tartaric Acid , Tetrasodium Edetate, Titanium Dioxide, Urea, Water.
Principal Display Panel - Cold and Hot Patch Box Label
NDC 66902-218-05
MAXIMUM STRENGTH
LIDOCAINE
Cold & Hot
Patch
UP TO
12
HOURS
MEDICATED PAIN RELIEF
WITH MENTHOL
LIDOCAINE 4%
MENTHOL 1%
- Desensitizes
aggravated nerves - Easy to apply
- Fast acting
Topical Anesthetic
Topical Analgesic
Package Contains 5 individually
wrapped Patches
5 PATCHES
3 5/16" x 5 1/2" (10 cm x 14 cm)
Principal Display Panel - Cold and Hot Patch Label
CVS
Health™
Compare to the active ingredients
in IcyHot® Lidocaine Patch
Plus Menthol*
NDC 66902-218-05
MAXIMUM STRENGTH
LIDOCAINE
Cold & Hot
Patch
MEDICATED PAIN RELIEF
WITH MENTHOL
LIDOCAINE 4%
MENTHOL 1%
- Desensitizes
aggravated nerves - Easy to apply
- Fast acting
Topical Anesthetic
Topical Analgesic
1 PATCH
3 5/16" x 5 1/2" (10 cm x 14 cm)