CAUTION: Keep out of reach of children
If swallowed, get medical help or contact Poison Control Center right away.
Warnings
For external use only. Flammable, keep away from fire or flame.
Do not use
in the eyes.
Stop use
If irritation, redness or other symptoms develop.
Ask a doctor
If condition persists or gets worse.
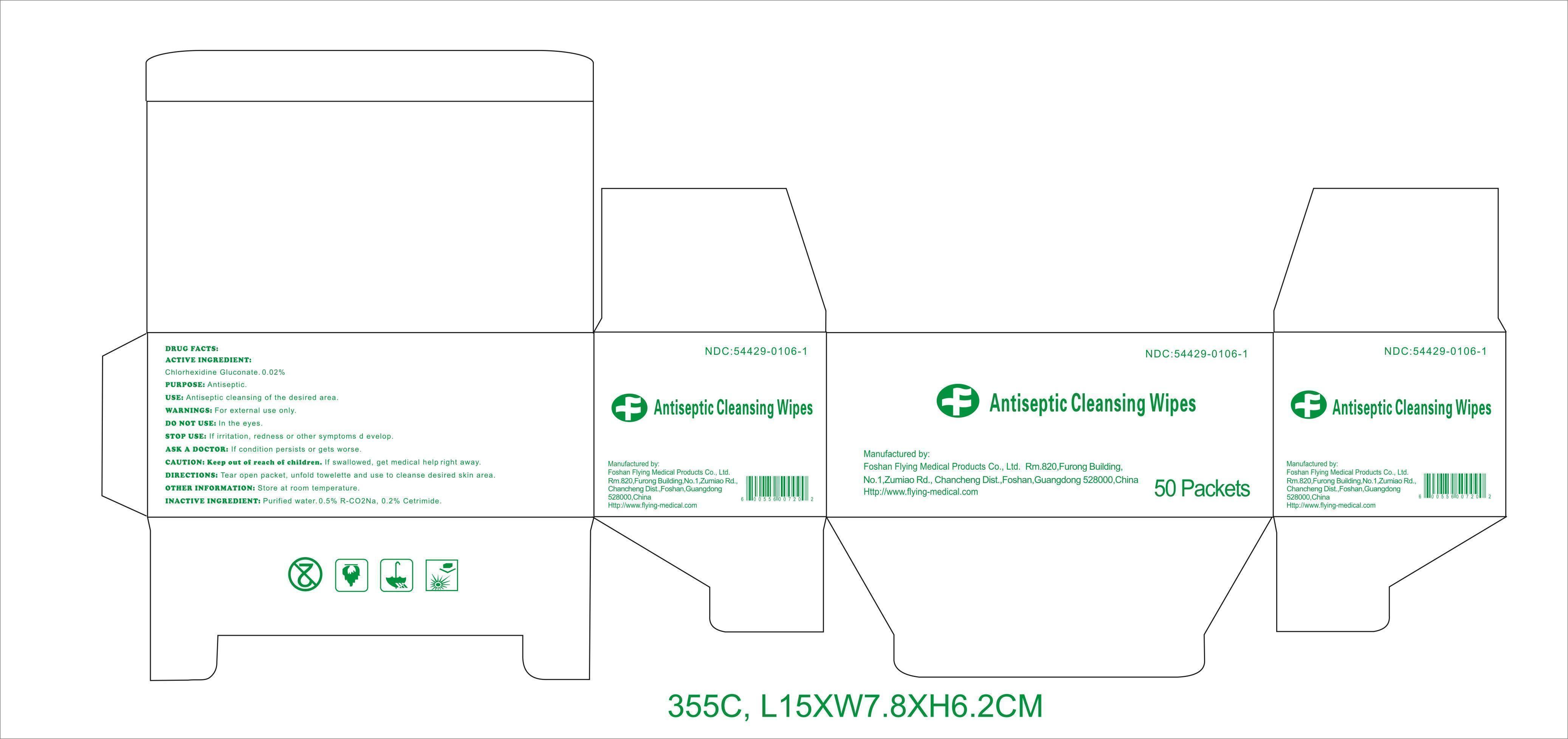
DRUG FACTS
Active ingredient
Chlorhexidine Gluconate 0.02%
Purpose
Antiseptic
Use
Antiseptic cleansing of the desired area.
Warnings
For external use only.
Do notuse
﹒in the eyes.
Stop use
If irritation, redness or other symptoms develop.
Ask a doctor
If condition persists or gets worse.
CAUTION: Keep out of reach of children.
If swallowed, get medical help right away.
Directions
Tear open packet, unfold towelette, and use to cleanse desired skin area.
Other information: Store at room temperature.
Inactive ingredients
Purified water, 0.5% R-CO 2Na, 0.2% Cetrimide