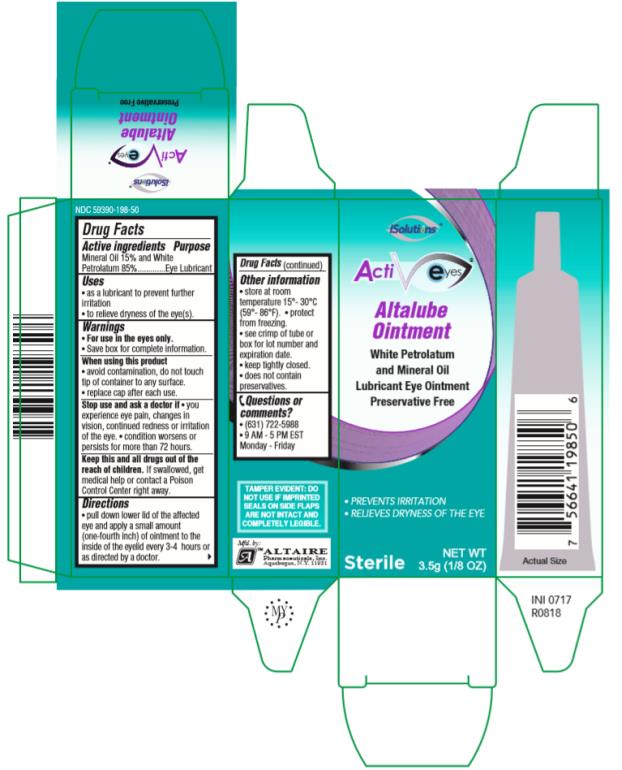
ActivEyes
Altalube Ointment 3.5g
NDC 59390-198-50
Drug Facts
Active ingredients
Mineral Oil 15% and White Petrolatum 85%
Uses
• as a lubricant to prevent further irritation • to relieve dryness of the eye(s).
Warnings
• Save box for complete information.
• For use in the eyes only.
When using this product
• avoid contamination, do not touch tip of container to any surface.
• replace cap after each use.
Stop use and ask a doctor if
• you experience eye pain, changes in vision, continued redness or irritation of the eye. • condition worsens or persists for more than 72 hours.
Keep this and all drugs out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• pull down lower lid of the affected eye and apply a small amount (one-fourth inch) of ointment to the inside of the eyelid every 3-4 hours or as directed by a doctor.
Other information
• store at room temperature 15°-30°C (59°-86°F). • protect from freezing.
• see crimp of tube or box for lot number and expiration date.
• keep tightly closed.
• does not contain preservatives.
Inactive Ingredients
None remove
Questions or comments?
• (631) 722-5988• 9AM – 5 PM EST Monday- Friday
PRINCIPAL DISPLAY PANEL
ActiEyes Altalube OintmentWhite Petrolatum and Mineral OilLubricant Eye Ointment Preservative FreeSterileNET WT3.5g (1/8 OZ)
Altaire Pharmaceuticals Inc.
